
Solubility Patterns
Double Replacement Reactions
Introduction
The alkaline earth metals are so-named because their oxides are highly basic (alkaline) and because they occur abundantly
on Earth. Alkaline earth metal compounds—carbonates, sulfates, silicates—are abundant in minerals such as dolomite
(calcium and magnesium carbonate), epsomite (magnesium sulfate), and baryte (barium sulfate). The solubilities of these
compounds vary widely, depending on the metal cation. Are there any patterns or periodic trends in the solubility behavior
of alkaline earth metal compounds?
Concepts
• Alkaline earth metals • Periodic trends • Solubility rules
• Double-replacement reactions • Ionic compounds
Materials
Alkaline earth metal chlorides Testing Solutions
Barium chloride solution, 0.1 M, BaCl
2
, 6 mL Ammonium oxalate solution, 0.25 M, (NH
4
)
2
C
2
O
4
,
4 mL
Calcium chloride solution, 0.1 M, CaCl
2
, 6 mL Potassium iodate solution, 0.2 M, KIO
3
, 4 mL
Magnesium chloride solution, 0.1 M, MgCl
2
, 6 mL Sodium carbonate solution, 1 M, Na
2
CO
3
, 4 mL
Strontium chloride solution, 0.1 M, SrCl
2
, 6 mL Sodium sulfate solution, 1 M, Na
2
SO
4
, 4 mL
Reaction plate, 24-well
Safety Precautions
Potassium iodate is moderately toxic and is irritating to skin, eyes, and the respiratory tract. Strontium and barium compounds are
toxic by ingestion. Oxalates are toxic by ingestion and are irritating to body tissues. Avoid contact of all chemicals with eyes and skin.
Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Please review current Material Safety Data
Sheets for additional safety, handling, and disposal information. Wash hands thoroughly with soap and water before leaving the
laboratory.
Procedure
1. Place a 24-well reaction plate onto the stage of the overhead projector and turn on the projector. Note that each well
is identified by a unique combination of a letter and a number, where the letters A–D refer to horizontal rows and the
numbers 1–6 refer to vertical columns.
2. Pass out the Solubility Patterns Worksheet. Instruct students to fill out the top part of the worksheet with observations
of what is occuring in the reaction wells during the demonstration. Have them use the abbreviations PPT and NR to
note the formation of a precipitate or no reaction, respectively.
3. Identify each horizontal row with the correct alkaline earth metal chloride and each vertical column with the correct
testing solution.
© 2016 Flinn Scientific, Inc. All Rights Reserved. 1
Publication No. 95009
061616
SCIENTIFIC
Solubility Patterns continued
2
© 2016 Flinn Scientific, Inc. All Rights Reserved.
4. Add 1 mL (about 25 drops or fill the well about
1
⁄4 inch or 0.5 cm deep) of an alkaline earth metal chloride solution to each
well in a horizontal row, as follows (see Figure 1):
• magnesium chloride to wells A1–A5
• calcium chloride to wells B1–B5
• strontium chloride to wells C1–C5
• barium chloride to wells D1–D5
5. Add 1 mL (about 25 drops) of testing solution to each
well in a vertical column, as follows (see Figure 1):
• potassium iodate to wells A1–D1
• sodium sulfate to wells A2–D2
• ammonium oxalate to wells A3–D3
• sodium carbonate to wells A4–D4
Note: The fifth column serves as a control
to identify the absence of a precipitate.
6. Students may now fill out the rest of the Solubility Patterns Worksheet and the Net Ionic Equation Worksheet.
Disposal
Please consult your current Flinn Scientific Catalog/Reference Manual for general guidelines and specific procedures governing
the disposal of laboratory waste. Barium compounds may be disposed of according to Flinn Suggested Disposal Method #27h.
All other solutions can be flushed down the drain with excess water according to Flinn Suggested Disposal Method #26b.
Tips
• Copy the student worksheet for students to fill out during the demonstration.
• This demonstration provides an excellent exercise for writing and balancing chemical equations and net ionic equations.
A second handout (Net Ionic Equation Worksheet) is included for this activity.
• The demonstration can be extended to include the identification of an unknown. Carry an unknown solution containing
one or two different alkaline earth metal cations through the sequence of precipitation reactions. Use the resulting
solubility pattern to identify the unknown cation(s).
• Strontium chloride forms a precipitate with carbonate but there is not as much precipitate as with other alkaline earth
metals.
• Discuss the relationship between the solubility pattern of alkaline earth metal compounds and hard water. Hard water
contains relatively high concentrations of magnesium and calcium ions. The problems caused by hard water range from
a nuisance (soaps leave soap scum, calcium stearate; detergents are not effective) to costly industrial “boiler scale” water
treatment programs (to eliminate the formation of calcium carbonate).
Discussion
Periodic trends are observed in the solubility of alkaline earth metal compounds. Although their chlorides and nitrates are all
water-soluble, alkaline earth metal compounds with other anions do not always dissolve in water. The solubility of alkaline
earth metal compounds with different anions is tested by carrying out double–replacement reactions. Reaction of calcium chloride
with sodium carbonate, for example, leads to an exchange of anions between the two metals to give calcium carbonate, which is
relatively insoluble in water and precipitates out as a solid when the two solutions are mixed. The chemical equation for this
reaction is given in Equation 1, where the abbreviations (aq) and (s) refer to aqueous solutions and solid precipitates,
respectively.
CaCl
2
(aq) + Na
2
CO
3
(aq) → CaCO
3
(s) + 2NaCl(aq) Equation 1
calcium chloride sodium carbonate calcium carbonate sodium chloride
The solubility of alkaline earth metal compounds decreases as you go down the column in the periodic table, i.e., solubility
A1
B1
C1
D1
A2
B2
C2
D2
A3
B3
C3
D3
A4
B4
C4
D4
A5
B5
C5
D5
A6
B6
C6
D6
IO
3
–
SO
4
2–
C
2
O
4
2–
CO
3
2–
control
MgCl
2
CaCl
2
SrCl
2
BaCl
2
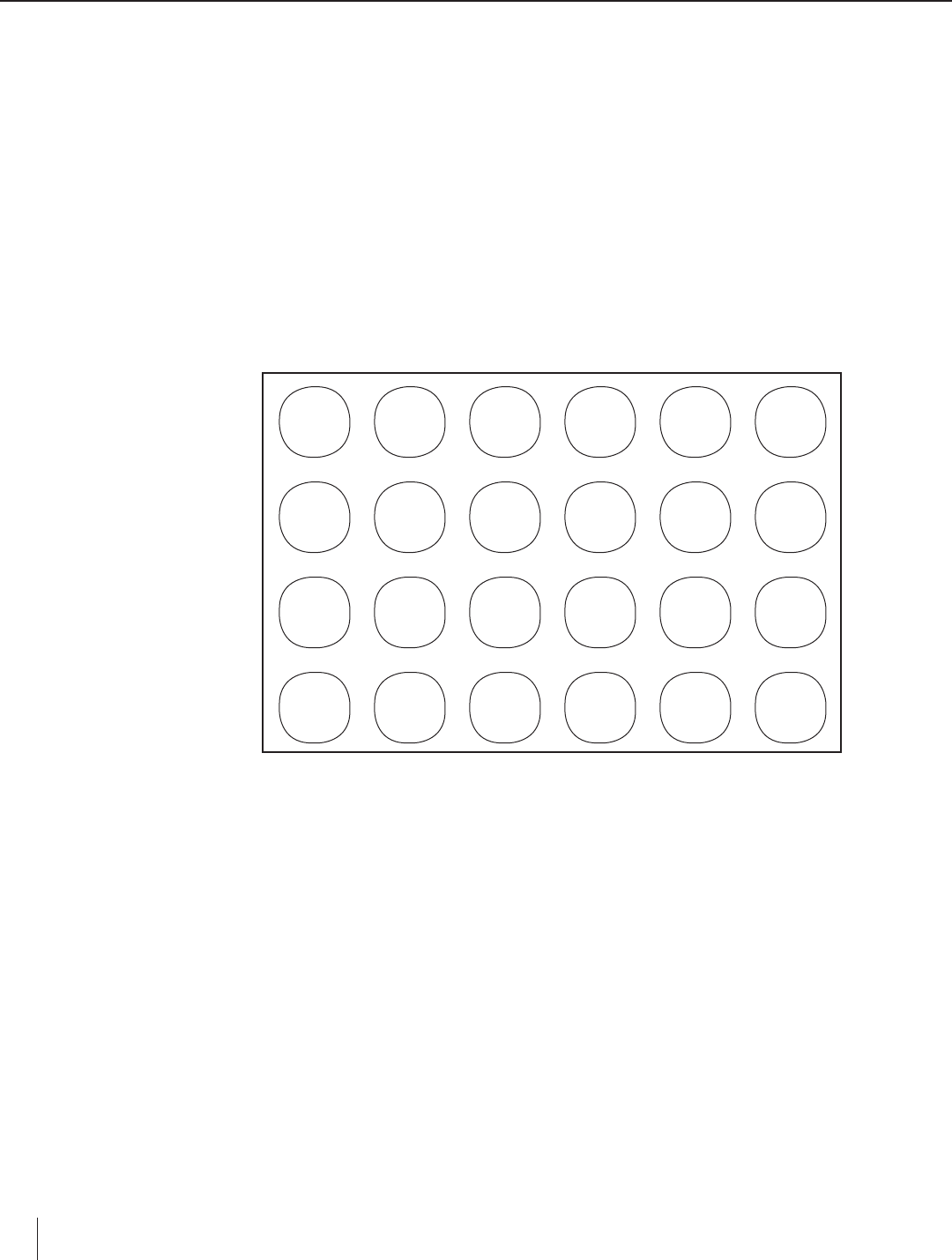
Figure 1. Demonstration Setup
Solubility Patterns continued
3
© 2016 Flinn Scientific, Inc. All Rights Reserved.
decreases as the atomic mass of the alkaline earth metal increases.
Connecting to the National Standards
This laboratory activity relates to the following National Science Education Standards (1996):
Unifying Concepts and Processes: Grades K–12
Systems, order, and organization
Evidence, models, and explanation
Content Standards: Grades 9–12
Content Standard B: Physical Science, structure and properties of matter, chemical reactions
Answers to Solubility Patterns Worksheet
1. Observe the reactions that develop in the reaction plate and have students record the results in the table of circles. Use the
abbreviations PPT and NR to note the formation of a precipitate or no reaction, respectively.
2. What patterns or trends are obvious in the solubility behavior of the alkaline earth metal compounds?
Which alkaline earth metal ion formed the most precipitates? Barium
The fewest? Magnesium
Which testing solution gave the most precipitates? Carbonate
The fewest? Iodate
3. Ask students to identify any periodic trend in the solubility behavior of alkaline earth metal compounds. Is there any
relationship between the solubility of alkaline earth metal compounds and the position of the metal in the periodic table?
Solubility decreases as you proceed down a family in the periodic table.
4. Propose an explanation for the observed solubility pattern.
The size or radius of an atom increases as one goes down a row (family) in the periodic table. For cations, the charge density (charge per
unit volume) will therefore decrease going down a row. The solubility of metal cations in water is strongly influenced by the hydration
of the positive ions by the polar water molecules. The hydration energy of an ion represents the change in energy that occurs when water
molecules attach to the cation. Water molecules are more attracted to a cation with a high charge density (i.e., a smaller atom) than one
that is larger with a lower charge density. Therefore, as one moves down a family of elements in the periodic table, the charge density
will decrease as will the solubility.
IO
3
–
SO
4
2–
C
2
O
4
2–
CO
3
2–
control
MgCl
2
CaCl
2
SrCl
2
BaCl
2
Figure 1. Demonstration Setup
A1
B1
C1
D1
A2
B2
C2
D2
A3
B3
C3
D3
A4
B4
C4
D4
A5
B5
C5
D5
A6
B6
C6
D6
NR
NR
NR
PPT
NR
NR
PPT
PPT
NR
PPT
PPT
PPT
PPT
PPT
PPT
PPT
X
X
X
X
NR
NR
NR
NR

Solubility Patterns continued
4
© 2016 Flinn Scientific, Inc. All Rights Reserved.
5. Use the observed solubility pattern to predict a chemical method for the separation of a mixture of calcium and barium
ions in solution. (Imagine a solution that is 0.1 M in both CaCl
2
and BaCl
2
. What reagents can be added to this mixture
and in what order to separate the two compounds?)
First, add potassium iodate solution to precipitate barium iodate. Filter the solution to isolate barium iodate. Then, add sodium carbonate
solution to precipitate calcium carbonate. Filter the solution to isolate calcium carbonate.
Answers to Net Ionic Equation Worksheet
Write out the net ionic equation for each reaction. If no reaction occurs, write NR.
1. MgCl
2
and KIO
3
NR
2. MgCl
2
and Na
2
SO
4
NR
3. MgCl
2
and (NH
4
)
2
C
2
O
4
NR
4. MgCl
2
and Na
2
CO
3
Mg
2+
(aq) + CO
3
2–
(aq) → MgCO
3
(s)
5. CaCl
2
and KIO
3
NR
6. CaCl
2
and Na
2
SO
4
NR
7. CaCl
2
and (NH
4
)
2
C
2
O
4
Ca
2+
(aq) + C
2
O
4
2–
(aq) → CaC
2
O
4
(s)
8. CaCl
2
and Na
2
CO
3
Ca
2+
(aq) + CO
3
2–
(aq) → CaCO
3
(s)
9. SrCl
2
and KIO
3
NR
10. SrCl
2
and Na
2
SO
4
Sr
2+
(aq) + SO
4
2–
(aq) → SrSO
4
(s)
11. SrCl
2
and (NH
4
)
2
C
2
O
4
Sr
2+
(aq) + C
2
O
4
2–
(aq) → SrC
2
O
4
(s)
12. SrCl
2
and Na
2
CO
3
Sr
2+
(aq) + CO
3
2–
(aq) → SrCO
3
(s)
13. BaCl
2
and KIO
3
Ba
2+
(aq) + 2IO
3
–
(aq) → Ba(IO
3
)
2
(s)
14. BaCl
2
and Na
2
SO
4
Ba
2+
(aq) + SO
4
2–
(aq) → BaSO
4
(s)
15. BaCl
2
and (NH
4
)
2
C
2
O
4
Ba
2+
(aq) + C
2
O
4
2–
(aq) → BaC
2
O
4
(s)
16. BaCl
2
and Na
2
CO
3
Ba
2+
(aq) + CO
3
2–
(aq) → BaCO
3
(s)
Flinn Scientific—Teaching Chemistry
™
eLearning Video Series
A video of the Solubility Patterns activity, presented by Irene Cesa, is available in Double Replacement Reactions and in Precipitation
Reactions and Solubility Rules, part of the Flinn Scientific—Teaching Chemistry eLearning Video Series.
Solubility Patterns continued
5
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Materials for Solubility Patterns are available from Flinn Scientific, Inc.
Materials required to perform this activity are available in the Solubility Patterns—Chemical Demonstration Kit available from
Flinn Scientific. Materials may also be purchased separately.
Catalog No. Description
AP6353 Solubility Patterns— Chemical Demonstration Kit
AP1447 Reaction plate, 24-well
B0144 Barium Chloride Solution, 0.1 M, 500 mL
C0234 Calcium Chloride Solution, 0.1 M, 500 mL
M0121 Magnesium Chloride Solution, 0.1 M, 500 mL
S0255 Strontium Chloride Solution, 0.1 M, 500 mL
A0198 Ammonium Oxalate Solution, 0.25 M, 500 mL
P0168 Potassium Iodate Solution, 0.2 M, 500 mL
S0234 Sodium Carbonate Solution, 1 M, 500 mL
S0352 Sodium Sulfate Solution, 1 M, 500 mL
Consult your Flinn Scientific Catalog/Reference Manual for current prices.
Solubility Patterns continued
6
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Solubility Patterns Worksheet
1. Observe the reactions that develop in the reaction plate and record the results in the table of circles. Use the abbreviations
PPT and NR to note the formation of a precipitate or no reaction, respectively.
2. What patterns or trends are obvious in the solubility behavior of the alkaline earth metal compounds?
a. Which alkaline earth metal ion formed the most precipitates?
b. The fewest?
c. Which testing solution gave the most precipitates?
d. The fewest?
3. Identify any periodic trend in the solubility behavior of alkaline earth metal compounds. Is there any relationship between
the solubility of alkaline earth metal compounds and the position of the metal in the periodic table?
4. Propose an explanation for the observed solubility pattern.
5. Use the observed solubility pattern to predict a chemical method for the separation of a mixture of calcium and barium
ions in solution. (Imagine a solution that is 0.1 M in both CaCl
2
and BaCl
2
. What reagents can be added to this mixture
and in what order to separate the two compounds?)
A1
B1
C1
D1
A2
B2
C2
D2
A3
B3
C3
D3
A4
B4
C4
D4
A5
B5
C5
D5
A6
B6
C6
D6
IO
3
–
SO
4
2–
C
2
O
4
2–
CO
3
2–
control
MgCl
2
CaCl
2
SrCl
2
BaCl
2
X
X
X
X

Solubility Patterns continued
7
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Net Ionic Equation Worksheet
Write out the net ionic equation for each reaction. If no reaction occurs, write NR.
1. MgCl
2
and KIO
3
2. MgCl
2
and Na
2
SO
4
3. MgCl
2
and (NH
4
)
2
C
2
O
4
4. MgCl
2
and Na
2
CO
3
5. CaCl
2
and KIO
3
6. CaCl
2
and Na
2
SO
4
7. CaCl
2
and (NH
4
)
2
C
2
O
4
8. CaCl
2
and Na
2
CO
3
9. SrCl
2
and KIO
3
10. SrCl
2
and Na
2
SO
4
11. SrCl
2
and (NH
4
)
2
C
2
O
4
12. SrCl
2
and Na
2
CO
3
13. BaCl
2
and KIO
3
14. BaCl
2
and Na
2
SO
4
15. BaCl
2
and (NH
4
)
2
C
2
O
4
16. BaCl
2
and Na
2
CO
3
