Inorganic chemistry II
Second stage / First semester
The Eighth lecture 2021/2022 Pro. Dr. Mohammed Hamid
Alkaline Earth Metals and their Compounds(Group IIA or 2, ns²)
POSITION OF ALKALINE EARTH METALS IN PERIODIC TABLE
The group IIA of the periodic table consists of six elements-beryllium, magnesium, calcium,
strontium, bariums and radium. These elements are collectively called as alkaline earth metals
because their earths (the old name for oxide) are basic (alkaline) and group IIA is known as
alkaline earth group. The oxides of three principal members calcium strontium and barium were
known much earlier than the metals themselves. These oxides were alkaline in nature and existed
in the earth and were named alkaline earths. The metals when discovered were also called alkaline
earths. This term is now applied to all the six elements of group IIA.
The first member beryllium is less active than other members and shows some abnormal properties
like lithium in 1A group. However, it shows resemblance with aluminium (a member of Iird
group). i.e. diagonal relationship. The last member, radium is radioactive in nature. Each member
of this group occupies a place just after the members of IA group in various periods of periodic
table except first period.
IA Li 3 Na 11 K 19 Rb 37 Cs 55 Fr 87
IIA Be 4 Mg 12 Ca 20 Sr. 38 Ba 36 Ra 88
The members of this group show a marked resemblance in their properties and possess same
electronic configuration. There is gradual gradation in the properties with the increase of atomic
number. This justifies their inclusion in the same group of periodic table. The main properties are
discussed below for this justification.
Electronic Configuration
The valence electron configuration of the atoms of the group IIA elements is ns
2
, where n is the
period number. The arrangement or the distribution of electron on various subshells in the atoms
of alkaline earth metals is given below
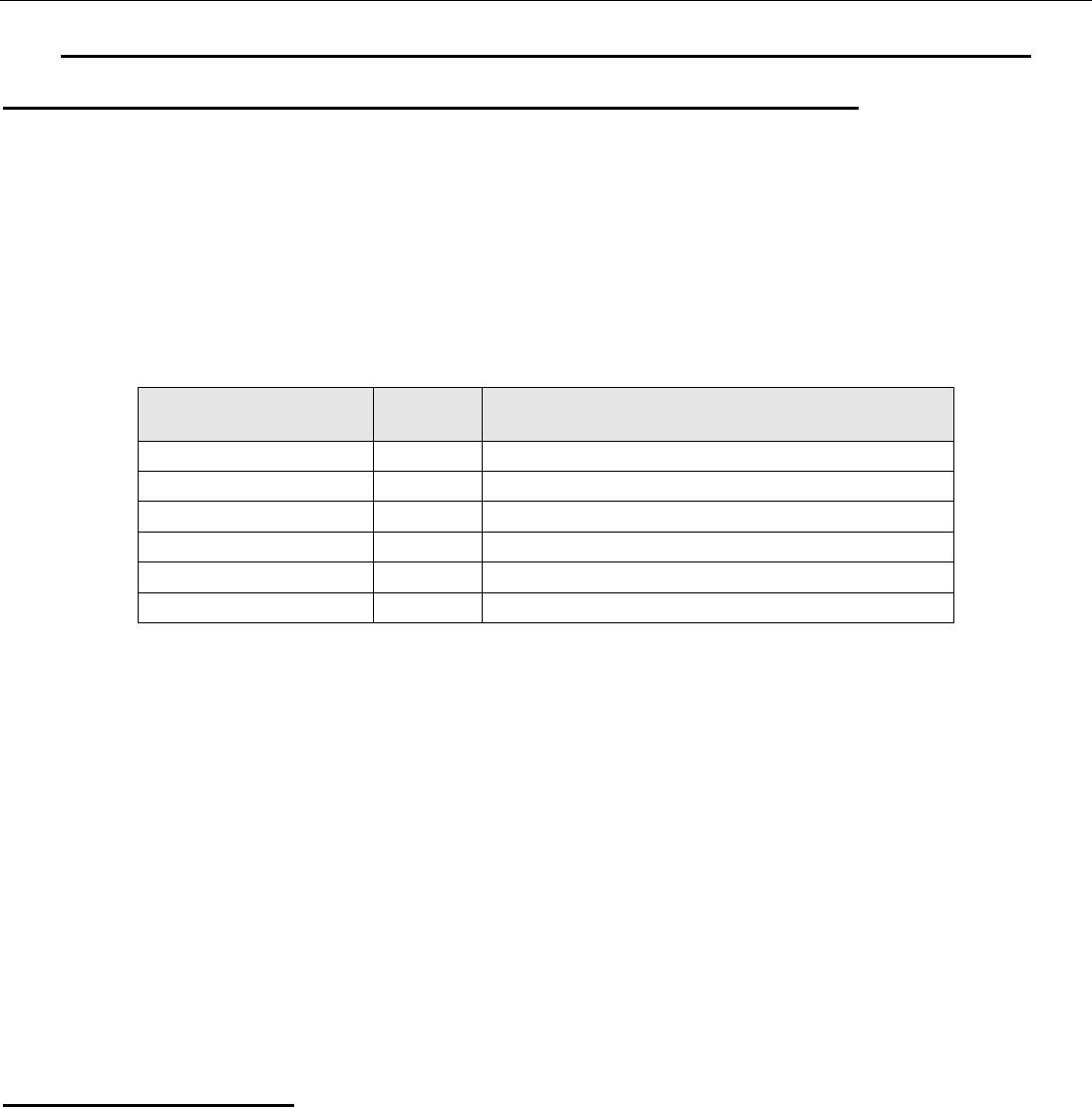
Element
Symbol
The most important minerals
Beryllium
Be
6
)
3
(SiO
2
AL
3
Be
Magnesium
Mg
O
2
.6H
2
KCl.MgCl
Calcium
Ca
O
2
.2H
4
,CaSO
3
.CaCO
3
MgCO
Strontium
Sr
3
SrCO
Barium
Ba
3
, BaCO
4
BaSO
Radium
Ra
It is found in uranium ores
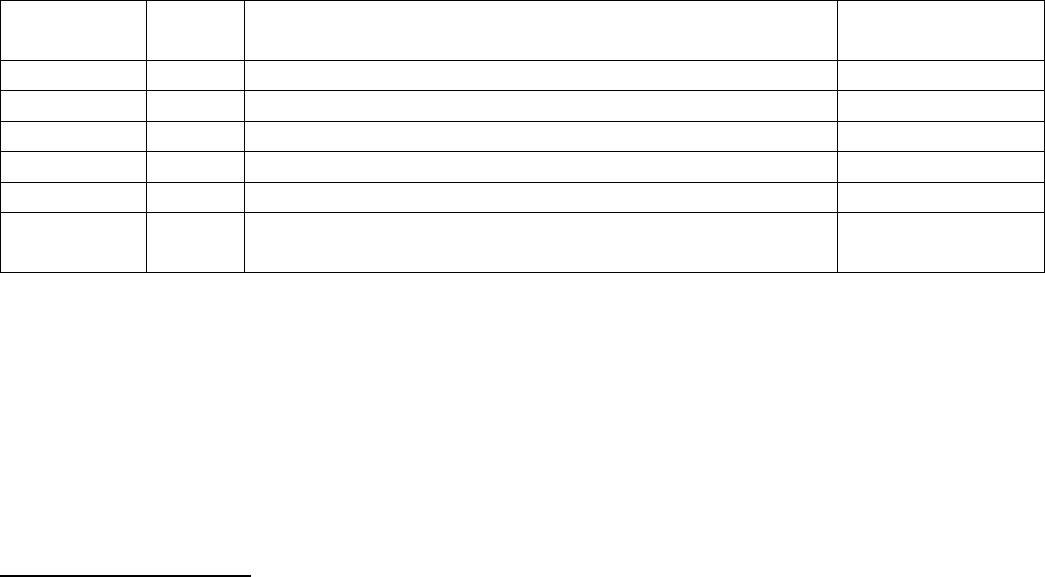
Element
At. No.
Electronic Configuration
Configuration of
the valency shell
Beryllium
4
1s
2
2s
2
[He]2s
2
Magnesium
12
1s
2
2s
2
2p
6
3s
2
[Ne]3s
2
Calcium
20
1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
[Ar]4s
2
Strontium
38
1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
6
5s
2
[Kr]5s
2
Barium
56
1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
6
4d
10
5s
2
5p
6
6s
2
[Xe]6s
2
Radium
88
1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
6
4d
10
4f
14
5s
2
5p
6
5d
10
6s
2
6p
6
7s
2
[Rn]7s
2
The outermost shell of these elements has two electrons and the penultimate shell contains 8
electrons except the first member which contains 2 electrons. Since, the last electron enters ns
orbital, these are s-block elements. Beryllium shows somewhat abnormal properties as its
electronic configuration is slightly different than the rest of the members. Because of their
similarity in electronic configuration [noble gas] ns², they are included in the same group, i.e., IIA
of the periodic table and closely resemble each other in the physical and chemical properties.
2. Physical Properties
(a) Physical state: All the group IIA elements are metals and too reactive, so that cannot occur in
the uncombined state in nature. They are all silvery white metals. They have greyish white lustre
when freshly cut, but tarnish soon after their exposure in air due to surface oxidation.
They are soft in nature but harder than alkali metals because metallic bonding is stronger than 1A
elements due to possession of 2 valency electrons. However, hardness decreases with increase in
atomic number.
(b) Atomic and ionic radii: The size of the atom increases gradually from Be to Ra, on account of
the presence of an extra energy shell at each step. The atoms are large but smaller han
corresponding IA elements since the extra charge on the nucleus attracts the electron cloud
inwards. Their ions are also large and size of the ion increases from Be
2+
to Ra
2+
Atomic volume also increases as the atomic number increases
(c) Density: These metals are denser than alkali metals in the same period because these can be
packed more tightly due to their greater nuclear charge and smaller size. The density decreases
slightly up to calcium and then increases considerably up to radium. Irregular trend is due to the
difference in the crystal structure of these elements.
(d) Melting and boiling points: The melting and boiling points of these elements are higher than
corresponding alkali metals. This is due to the presence of two electrons in the valency shell and
thus, strongly bonded in the solid state. However, melting and boiling points do not show any
regular trend because atoms adopt different crystal structures.
(e) Ionisation energies and electropositive character: The first and second ionisation energies of
these metals decrease from Be to Ba. The second ionisation energy in each case is higher than the
first, nearly double the first ionisation energy.
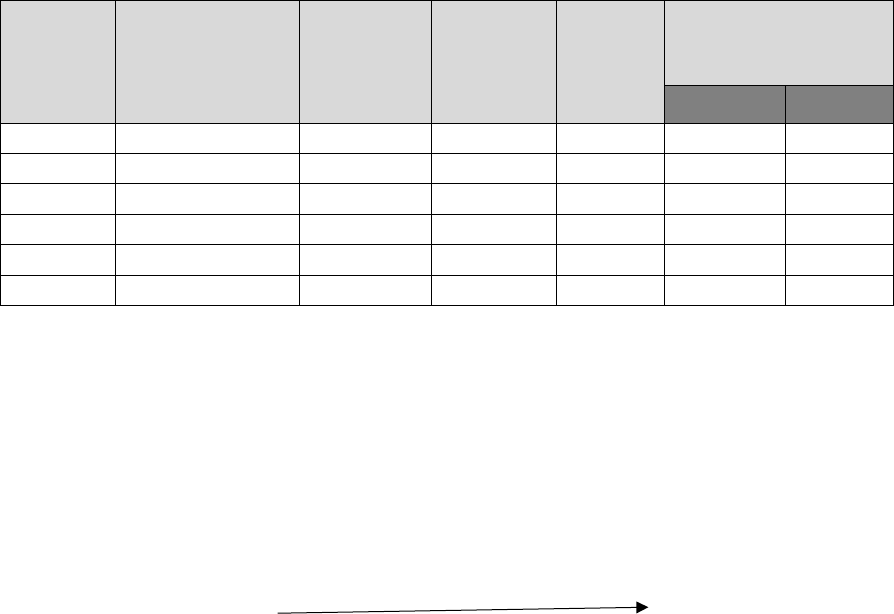
The ionisation energy of last member, radium, is slightly higher than that of barium and it is
difficult to explain this anomalous behaviour.
Symbol
Abundance
in earth
’
s crust
p.p.m
Atomic
radius A
0
Ionic
radius A
0
Density
g/cc
Ionization
potentials
1
st
eV
2
nd
eV
Be
6
0.89
0.31
1.8
9.3
18.2
Mg
20.9
1.36
0.65
1.7
7.6
15.0
Ca
36.3
1.74
0,99
1.6
6.1
11.9
Sr
300
1.91
1.13
2.6
5.7
11.0
Ba
250
1.98
1.35
3.5
5.2
10.0
Ra
1.3x10
-4
-
1.50
5.0
5.3
10.1
Although, the ionisation energies of these elements are higher than those of alkali metals, yet these
are sufficiently low to make these atoms to lose two electron of their valency shell to form M
2+
ions and achieve the inert gas configuration. These metals are thus, strongly electropositive in
nature but less than corresponding alkali metals. The electropositive character increases from Be
to Ba. Metallic character and reactivity are directly linked with the tendency to lose electron or
electrons, te with electropositive nature. Thus, these characters increase gradually from Be to Ba.
Be Mg Ca Sr Ba Ra
Electropositive nature increases Metallic
character increases Reactivity of the metals increases
(f) Oxidation states: The alkaline earth metals form a basic oxide with general formula RO. All
show a stable oxidation state +2 in their compounds. The second ionisation energy is nearly double
the first ionisation energy for all these elements. This should cause these elements to exhibit a
stable +1 oxidation state and form compounds like BaCl , SrBr , Cal etc., instead of BaCl
2
, SrBr
2
,
Cal
2
, etc. However, the lattice energy increases as the charge on the ion increases. The increase in
the lattice energy on account of the second electron from ns² is much more than the energy required
(second ionisation energy) to remove it. Hence, the stability of +2 oxidation state is due to high
lattice energy. The second factor responsible for +2 oxidation state is the hydration energy which
is high for M
2+
ions. On account of the availability of energy, the process does not stop to M
+
state
but reach to M
2+
state readily.
Since, the bivalent ions, M
2+
, have an inert gas configuration, it is very difficult to remove the
third electron and hence oxidation state higher than +2 is not possible.
Amongst alkaline earth metals, beryllium has the highest ionisation energy, i.e., least
electropositive in nature. Thus, beryllium has the minimum tendency to form Be
2+
ion and hence
a number of compounds of beryllium are covalent in nature.
(g) Hydration of ions and hydration energy: The M
2+
jons of alkaline earth metals are extensively
hydrated to form hydrated ions, [M(H₂O)
x
]
2+
and during hydration a huge amount of energy, called
hydration energy, is released.
M²+ + xH₂O → [M(H₂O)
X
]
2+
+ Energy
The degree of hydration and the amount of hydration energy decreases as the size of the ion
increases from Be
2+
to Ba
2+
The hydration energies of alkaline earth metal ions are higher than those of alkali metal ions and
thus the compounds of alkaline earth metals are more extensively hydrated than alkali metals.
Magnesium chloride and calcium chloride exist as MgCl
2
6H₂O and CaCl
2
-6H₂O, respectively,
while sodium chloride and potassium chloride exist as NaCl and KCl.
The ionic mobilities or ionic conductance of these ions increase from [Be(H₂O)
x
]
2+
to
[Ba(H₂O)
x
]²+ because [Be(H₂O)
x
]
2+
becomes heavy due to high degree of hydration.
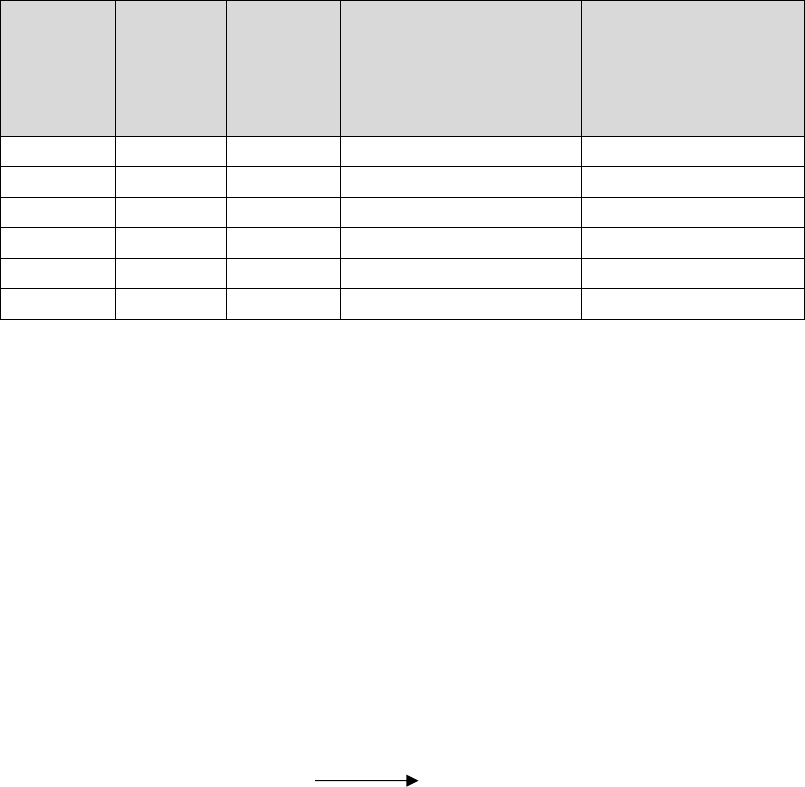
(h) Electronegativity : The tendency to attract electrons is low. The electronegativity values are
thus small and decrease from Be to Ra.
Symbol
m.pt.(K)
b.pt.(K)
Oxid. Potential
(Volt.)
Electronegativity
Be
1560
2745
1.97
1.5
Mg
924
1363
2.36
1.2
Ca
1124
1767
2.84
1.0
Sr
1062
1655
2.89
1.0
Ba
1002
2078
2.92
0.9
Ra
973
-
-
-
(i) Conductivity : On account of the presence of two loosely bond valency electrons per atom
which can move freely throughout the crystal lattice, the alkaline earth metals are good conductors
of heat and electricity.
(j) Flame colouration: In the case of Ca, Sr. Ba and Ra, the electrons can be excited by the supply
of energy to higher energy levels. When the excited electrons return to the original level, the
energy is released in the form of light. In beryllium and magnesium, the electrons are tightly held
and hence excitation is rather difficult, thus do not show flame colouration. Ca, Sr, Ba and Ra
impart a characteristic colour to the flame. Ca-brick red; Sr-crimson; Ba-green; Ra-crimson
(k) Reducing nature: The alkaline earth metals have the tendency to lose electrons and change into
bivalent cation:
M M
2+
+ 2e
Hence, they act as strong reducing agents. The reducing nature increases as the atomic number
increases.
Strength of a reducing agent is linked with the value coxidation potential. The values of the
oxidation potential increases from Be to Ba, hence the strength as a reducing agent increases in
the same order.
The oxidation potentials are lower than those of the alkali metals, hence, the alkaline earth metals
are weaker reducing agents than alkali metals. The reason for the lower values of oxidation
potentials is due to high heats of atomisation (sublimation) and ionisation energies.
(1) Colour and magnetic property: Since, the divalent ions have noble gas configuration with no
unpaired electrons, their compounds are diamagnetic and colourless unless the anion is coloured.
The metals are also diamagnetic in nature as all the orbitals are fully filled with spin paired
electrons, e.g.,
Chemical Properties
(a) Occurrence: Alkaline earth metals are reactive elements and hence do not occur free in nature.
Magnesium and calcium are found in abundance in nature. Beryllium is not
very abundant. Strontium and barium are much less abundant. Radium is a rare element.Calcium
and magnesium are the most common and commercially useful of the alkaline earth elements. We
can see in the table given below, calcium is the fifth and magnesium is the eighth most abundant
element in the earth's crust.
Ten most Abundant Elements in the Earths Crust
No.
Element
Mass percentage
No.
Element
Mass percentage
1
Oxygen
46.6
6
Sodium
2.8
2
Silicon
27.7
7
Potassium
2.6
3
Aluminum
8.3
8
Magnesium
2.1
4
Iron
5.1
9
Titanium
0.4
5
Calcium
3.6
10
Hydrogen
0.1
Like the alkali metals, the group IIA elements occur i nature as silicate rocks. They also occur as
carbonates an sulphates, and many of these are commercial sources of alkalin earth metals and
compounds.
These metals occur in nature largely as carbonates, sulphate and silicates.
(b) Extraction: The metals of this group are not easy to produce on account of following reasons:
(i) The metals cannot be produced by chemical reduction because they are themselves strong
reducing agents and they react with carbon and form carbides.
(ii) They are strongly electropositive and react with water and so aqueous solutions cannot be used
for displacing them with another metal.
(iii) The electrolysis of aqueous solutions of their salts produces hydrogen at cathode rather than
the metal as the metal reacts with water. Electrolysis of an aqueous solution can be carried out by
using mercury as cathode, but recovery of the metal from amalgam is difficult.
These metals are best isolated by electrolysis of their fused metal halides containing NaCl. NaCl
lowers the fusion temperature and makes the fused mass as good conductor of electricity.
(c) Reactivity towards water: Calcium, strontium, barium and radium decompose cold water
readily with evolution of hydrogen.
M + 2H₂O →M(OH)₂ + H₂
Magnesium decomposes boiling water but beryllium does not react with water, even when red hot,
its protective oxide film survives even at high temperature as its oxidation potential is lower than
the other members.
Reactivity of alkaline earth metals increases as we move down the group as the oxidation potential
increases. However, the reaction of alkaline earth metals is less vigorous than alkali metals.
(d) Reactivity towards atmosphere: Except beryllium, these metals are easily tarnished in air as a
layer of oxide is formed on their surface. The effect of atmosphere increases as the atomic number
increases. Barium in powdered form bursts into flame on exposure to air.
M + air →MO + M3N2
(Ca, Sr or Ba)
(e) Reactivity towards acids: Like alkali metals, the alkaline earth metals freely react with acids
and displace hydrogen.
M + H₂SO4 →MSO4 + H₂
M+2HC1 → MCl₂ + H₂
Beryllium behaves differently as it dissolves in caustic alkalies also with liberation of hydrogen.
It is due to diagonal relationship with aluminium. Be is thus amphoteric in nature.
Be + 2NaOH → Na₂BeO₂ + H₂
Sodium beryllate
(f) Affinity for non-metals: Alkaline earth metals have great affinity for non-metals. They
directly react with non-metals at the appropriate temperature.
(i) Reaction with hydrogen: Except beryllium, all combine with hydrogen directly to form
hydrides of the type MH₂ when heated with hydrogen.
M + H₂→ MH₂
BeH₂ and MgH₂ are covalent in nature while other hydrides are ionic in nature. Calcium, strontium
and barium hydrides liberate hydrogen at anode on electrolysis in the fused state. Ionic hydrides
are violently decomposed by water evolving hydrogen, CaH₂ is technically called hydrolith and
used on large scale for the production of hydrogen.
CaH₂ + 2H₂O→ Ca(OH)
2
+2H₂
[BeH₂ is not obtained by direct combination of beryllium and hydrogen. It is formed by reacting
beryllium chloride with lithium aluminium hydride.
2BeCl₂ + LiAIH
4
→ 2BeH₂ + LiCl + AlC13]
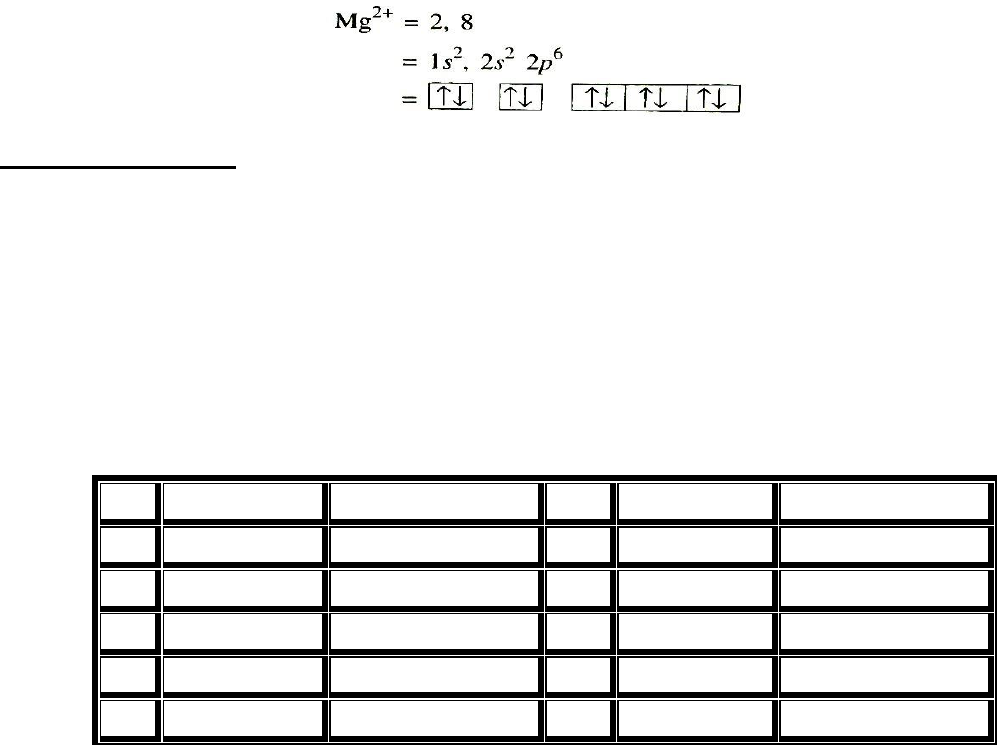
It is polymeric. (BeH₂), possesses hydrogen bridges. Three centre bonds are present in which a
banana shaped molecular orbital covers three atoms Be---H---Be and contains two electrons.
Hydrogen atoms lie in the plane perpendicular to the plane of molecule containing beryllium
atoms.
The stability of the hydrides decreases with increasing atomic number because the metallic nature
of the elements increases.
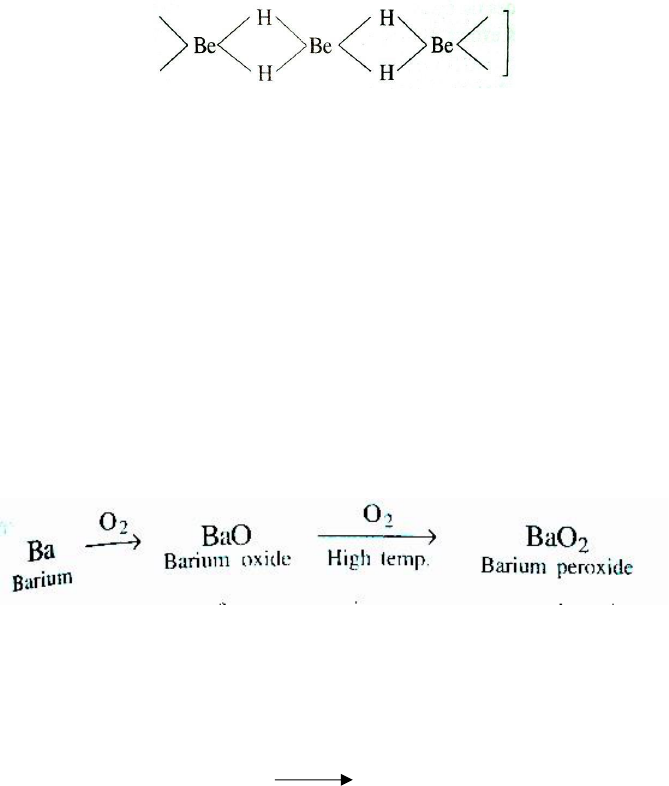
(ii) Reaction with oxygen (Oxides and Hydroxides): Except Ba and Ra, these elements when
burnt in oxygen form oxides of the type MO.
2M + O₂→ 2MO
Beryllium metal is relatively unreactive and does not react below 600
0
C. but the powder form is
much more reactive and burns brilliant. The element. Mg burns with dazzling brilliance evolving
a lot of heat.
Barium and radium, being highly electropositive, form peroxides.
Thus, the affinity for oxygen increases on moving down the group.
BeO is usually formed by ignition of the metal, but the other metal oxides (MO type) are usually
obtained by thermal decomposition of the carbonates, MCO
3
.
MCO
3
Heat
MO + CO₂
The oxides are very stable compounds (BeO and MgO are used as refractory materials) and white
crystalline solids. Except BeO (predominantly covalent), all the other oxides are ionic and possess
NaCl structure (face centred cubic). The reason for high stability is due to high lattice energy
values which, however, decrease as the size of the metal ion increases.
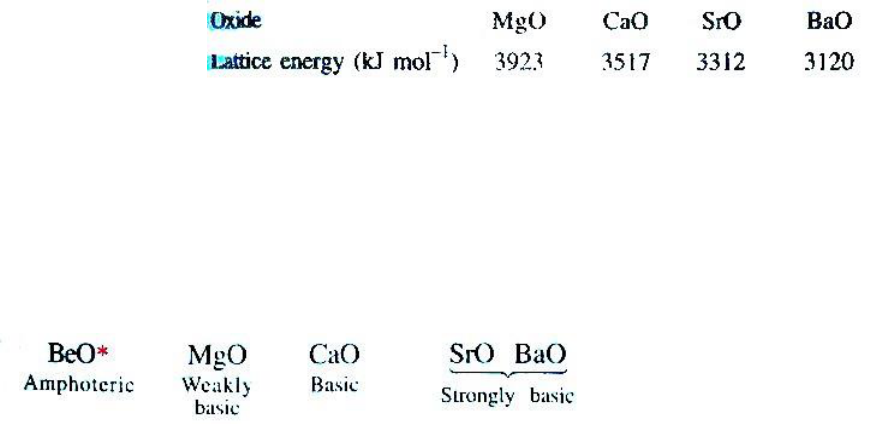
Except BeO, which is amphoteric in nature, other MO oxides are basic in nature as they combine
with water to form basic hydroxides. This reaction is highly exothermic.
MO + H₂O →M(OH)₂ + Heat
(where, M = Ca²+, Sr
2+
or Ba²+)
Basic nature of the oxides increases gradually from BeO to BaO.
(*The amphoteric nature is supported by its reaction with acids as well
BeO + 2HCl →BeCl₂ + H₂O. BeO+ 2NaOH → Na
2
BeO
2
+ H
2
O.)
[BeO and MgO are insoluble in water as these are tightly held together in the solid state.]
Be(OH)₂ is amphoteric, but the hydroxides of other alkaline earth metals are basic. The basic
strength increases gradually.
Be(OH)₂ + 2HCl → BeCl₂ + 2H₂O
Be(OH)2 + 2NaOH → Na₂BeO₂ + 2H₂O
Sod. beryllate
Be(OH)
2
+ 2OH
-
→ [Be(OH)
4
1
2-
Beryllate ion
The solubility of the hydroxides increases with increase of atomic number of the alkaline earth
metals. This is due to the fact that decrease in lattice energy is more than decrease in hydration
energy on moving down the group. The increasing solubility can also be explained on the basis of
values of their solubility products which increase from Be(OH)₂ to Ba(OH)₂. Be(OH)
2
and
Mg(OH)
2
are almost insoluble in water.
Metal hydroxide Be(OH)₂ Mg(OH)
2
Ca(OH)
2
Sr(OH)
2
Ba(OH)
2
Solubility product 1.6x10
-26
8.9x10
-12
1.3x10
-4
3.2x10
-4
5.4x10
-3
(K
sp
)
The hydroxides decompose on heating. The thermal stability increases from Be(OH)₂ to Ba(OH)
2
.
Mg(OH)₂ → MgO + H₂O
Ca(OH)
2
→ CaO + H₂O
(iii) Reaction with halogens (Halides): The alkaline earth metals directly combine with halogens,
when heated with them.
M + X₂
Heated
MX₂
(X₂ = F₂, Cl₂, B
2
, or I₂)
The alkaline earth metal halides can be obtained by the action of halogen acids on metals, their
oxides, hydroxides and carbonates.
M + 2HX MX
2
+ H
2
MO + 2HX MX
2
+ H
2
0
M(OH)
2
+ 2HX MX
2
+ 2 H
2
0
MCO
3
+ 2HX MX
2
+ H
2
0 + CO
2
Beryllium halides are covalent in nature. This is due to small size and high charge of Be²+ ion,
i.e., it has high polarising power. The glassy forms of halides are known to have chains of
---- X₂Be X₂Be----.
Cl Cl Cl
Be Be
Cl Cl Cl
The halides of the type MX₂ (fluorides, chlorides, bromides and iodides) of other metals are
ionic solids. The solubility of these halides decreases with increasing atomic number of the
metal as there is decrease in hydration energy with the increase in the size of the metal ion.
Solubility of BeF₂ will therefore be greater than BaF₂.
As the ionic character increases on moving down the group. the melting points and their
conductivity increase from magnesium halides to barium halides. They are good conductors in
molten state.
The halides are hygroscopic in nature and readily form hydrates, e.g., MgCl₂.6H₂O,
CaCl
2
.6H₂O, BaCl₂ 2H₂O, etc. Calcium chloride has a strong affinity for water and is used as a
dehydrating agent. However, BeCl₂ fumes in moist air due to its hydrolysis.
BeCl₂ + H₂O Be(OH)₂ + 2HCl
(iv) Reaction with nitrogen : All the alkaline earth metals burn in nitrogen to form nitrides of
the type M
3
N₂.
3M + N₂ M
3
N₂
The ease of formation of nitrides decreases from Be to Ba. This is in contrast to alkali metals
where only Li3N is formed. Because the N₂ molecule is very stable, it requires very high energy
to form N³ ions. The large amount of energy comes from the very large amount of lattice energy
evolved when the crystalline solid is formed. The lattice energy is particularly high because of the
high charges on the ions M
2+
and N
3-
Be
3
N₂ is volatile (covalent character) while other nitrides are not volatile as they are ionic
crystalline solids. The nitrides are hydrolysed with water liberating ammonia.
M
3
N₂ + 6H₂O 3M(OH)₂ + 2NH
3
(v) Reaction with carbon (Carbides): With the exception of Be, other metals when heated with
carbon in an electric furnace or when their oxides are heated with carbon form carbides of the type
MC₂. These carbides are called acetylides as on hydrolysis they evolve acetylene.
M + 2C MC
2
MO +3C MC
2
+ CO
MC
2
+ 2H
2
O M(OH)
2
+ C
2
H
2
MC₂ carbides, all have a distorted sodium chloride type of structure, M
2+
replaces Na
+
and
[ - C≡C- ]
2-
replaces Cl
-
.
MgC₂, on heating, changes into Mg₂C
3
. Mg
2
C
3
on hydrolysis evolves propyne, CH
3
-C≡CH methyl
acetylene).
Mg2C3+ 4H₂O 2Mg(OH)₂ + C3H4
When BeO is heated with carbon at about 2000°C, a brick red coloured carbide of formula, Be₂C,
is formed. This on hydrolysis evolves methane and is, thus, called methanide.
Be₂C+4H₂O 2Be(OH)₂ + CH₂
It is also ionic but possesses an antifluorite structure.
(vi) Reaction with sulphur and phosphorus : Alkaline earth metals directly combine with
sulphur and phosphorus when heated with them to form sulphides of the type MS and phosphides
of the type M
3
P
2
, respectively.
M+S MS
3M+2P M
3
P
2
Sulphides on hydrolysis liberate H₂S, while phosphides on hydrolysis evolve phosphine.
MS+ dil.acid → H₂S
M
3
P₂ + dil.acid → PH
3
Sulphides are phosphorescent. They cannot be precipitated by passing H₂S through their salts
solutions as they are decomposed by water.
2MS + 2H₂O M(OH)₂ + M(HS)2
(g) Nature of oxy salts: (i) Bicarbonates an carbonates: Bicarbonates of alkaline earth metals
do no exist in solid state but are known in solutions only. When such solutions are heated,
bicarbonates are decomposed with evolution of carbon dioxide.
M(HCO3)2
Heated
MCO3 + CO₂ + H₂O
(Solution)
Carbonates of alkaline earth metals (MCO3) are insoluble water. These dissolve in water in
presence of carbon dioxide
MCO3 + H₂O + CO₂→M (HCO3)2
Solubility of carbonates decreases on moving down t group, while stability increases. This is
evident from the values of decomposition temperatures of various carbonates which increase
gradually.
MCO
3
MO+CO
2
Decomposition BeCO
3
MgCO
3
CaCO
3
SrCO
3
BaCO3
temp. (°C) 100 540 900 1290 1360
Increasing stability can be explained on the basis of polarisation and covalent character. Be
2+
is
smallest in size hence show high polarising power. BeCO
3
is least ionic and has least stability.
BeCO3 < MgCO3 < CaCO3 < SrCO3 < BaCO3
Increasing ionic character and stability
The instability of BeCO3 is due to small size of Be
2+
ion which is unable to stabilise the bigger
CO
3
2-
ion. However, it can stabilise the smaller O
2-
ion. The stability of other carbonates increases
as the size of other cations increases gradually.
The carbonates are all ionic, but BeCO3 is unusual because it contains hydrated ion [Be(H₂O)
4
]
2+
rather than Be²+.
(ii) Sulphates: Alkaline earth metals form sulphates of the type MSO4. These are prepared by the
action of sulphuric acid on oxides, hydroxides or carbonates.

MO + H₂SO
4
→ MSO
4
+ H₂O
M(OH)₂ + H₂SO
4
→ MSO
4
+ 2H₂O
MCO3 + H₂SO4→ MSO
4
+ H₂O + CO₂
The solubility of sulphates decreases on moving down the group. CaSO
4
is sparingly soluble,
while SrSO
4
, BaSO
4
and RaSO
4
are almost insoluble. The solubilities of BeSO
4
and MgSO
4
are
due to high energy of solvation of smaller Be²+ and Mg²+ ions. The values of solubility products
which decrease gradually also explain the decrease in solubility on moving down the group.
Metal sulphate BeSO
4
MgSO
4
CaSO
4
SrSO
4
BaSO
4
Solubility product very high 10 2.4×10
-5
7.6x10
-7
1.5x10
-9
The sulphates decompose on heating to give the corresponding oxide (MO).
2 MSO
4
Heat
2 MO+2SO₂ +O₂
The stability increases as the basic nature of the meta increases. This is evident from the
decomposition temperatures
Metal sulphate BeSO
4
MgSO
4
CaSO
4
SrSO
4
Decomposition temp. (°C) 500 895 1149 1374
Sulphates are reduced into sulphides on heating wit carbon.
(iii) Nitrates: Alkaline earth metals form nitrates of the type M(NO
3
)
2
. These are prepared by the
action of nitric acid with oxides, hydroxides and carbonates.
Nitrates of these metals are soluble in water. On heating they decompose into their corresponding
oxides with evolution of a mixture of nitrogen dioxide and oxygen.
2M(NO3)
2
2MO+4NO₂+O₂
Beryllium also forms a basic nitrate in addition to the norm salt. Basic nitrate is a covalent
compound.
Be(NO
3
)
2
125°C
[Be O(NO₂).]
Basic beryllium nitrate
(h) Solutions of metals in liquid ammonia: Like alkali metals, alkaline earth metals also dissolve
in liquid ammonia to for coloured solutions. Dilute solutions are bright blue in colour due to
solvated electrons. These solutions decompose very slowly forming amides and evolving
hydrogen.
M M
2+
+ 2e
2NH3 + 2e → 2NH₂
1-
+ H₂
M
2+
+ 2NH₂
1-
→ M(NH₂)
2
When the solution is evaporated, hexammoniate, M(NH
3
)
6
is formed. These slowly decompose to
give amides.
M(NH
3
)
6
→ M(NH₂)
2
+ 4NH
3
↑+H₂ ↑
Concentrated solutions of the metals in ammonia are bronze coloured.
(i) Formation of amalgams: Alkaline earth metals combine with mercury to form amalgams.
(j) Complex formation: Generally, the alkaline earth metals do not form complexes. However,
the smaller ions have some tendency to form complexes. Beryllium forms stable complexes such
as [BeF
3
]
-
, [BeF
4
]
2-
and [Be(H₂O)
4
]
2+
Complexes of the type BeCl₂R
2
are formed where R is an
ether, aldehyde or ketone with an oxygen as a donor atom. Beryllium is unique in forming a series
of stable complexes of formula [Be
4
O(R)
6
] , where R may be NO
3
-
, HCOO
-
, CH
3
COO
-
, C
6
H
5
COO
-
, etc.
The most important complex formed by magnesium is chlorophyll in which magnesium is
bonded to the four heterocyclic nitrogen atoms. Calcium, strontium and barium form complexes
only with strong complexing agents like acetylacetone, EDTA, etc.
(k) Organo-metallic compounds : Both Be and Mg form an appreciable number of compounds
with M-C bonds but only a few are known for Ca, Sr and Ba. Grignard reagents are very important
in organic chemistry which can be used to form a wide variety of organic compounds.
Mg + RBr
Dry ether
RMgBr (R = alkyl or aryl)
Grignard reagents
BeCl₂ reacts with Grignard compounds forming reactive dialkyls and diaryls.
2RMgCl + BeCl₂
Ether
BeR₂ + 2MgCl₂
Dialkyls and diaryls of Mg, Ca, Sr and Ba can also be obtained by similar reactions.
SOLUBILITY OF COMPOUNDS OF ALKALINE EARTH METALS
In the case of the compounds of Ca, Sr and Ba the following facts are observed:
(i) The solubility of hydroxides, fluorides and oxalates increases from calcium to barium.
(ii) The solubility of carbonates, sulphates and chromates decreases from calcium to barium.
The solubility of an ionic compound depends on two factors: (i) lattice energy and (ii) hydration
energy. These two factors oppose each other. If lattice energy is high, the ions will be tightly
packed in the crystal and, therefore, solubility will be low. If hydration energy is high, the ions
will have greater ten dency to be hydrated and, therefore, the solubility will be high.
In the case of hydroxides, fluorides and oxalates the lattice energies are different, i.e., lattice
energy decreases as the size of the cation increases. This tends to increase the solubility as it
overcomes the counter effect of decrease in hydration energy. Hence, the solubility of the
hydroxides, fluorides and oxalates increases from Ca to Ba.
In the case of carbonates, sulphates and chromates the anions are large in size and small changes
in cation size do not alter the lattice energies, i.e., lattice energies are about the same. However,
the hydration energies decrease from Ca
2+
to Ba
2+
. Hence, the solubility of carbonates, sulphates
and chromates decreases from calcium to barium.
DIFFERENCE BETWEEN ALKALINE EARTH METALS AND ALKALI METALS
Both alkaline earth metals and alkali metals are s-block elements as the last differentiating
electron enters the ns-orbital. They resemble with each other in many respects but still there are
certain dissimilarities in their properties on account of different number of electrons in the valency
shell, smaller atomic radii, high ionisation potential, higher electronegativity, etc. The man points
of difference between alkaline earth metals and alkali metals are given below:
Properties
Alkaline earth metals
Alkali metals
(i) Electronic
configuration
Two electrons are present in the valency shell.
The con figurations is ns².
One electron is present in the valency shell. The
configuration is ns¹.
(ii) Valency
Bivalent.
Monovalent.
(iii) Electropositive nature
Less electropositive.
More electropositive.
(iv) Hydroxides
Weak bases, less soluble and decompose on
heating.
Strong bases, highly soluble and stable towards
heat.
(v) Bicarbonates
These are not known in free state. Exist only in
solution.
These are known in solid state.
(vi) Carbonates
Insoluble in water. Decompose on heating.
Soluble in water. Do not decompose on heating
(Li₂CO3 is an exception).
(vii) Action of nitrogen
Directly combine with nitrogen and form
nitrides.
Do not directly combine with nitrogen.
(viii) Action of carbon
Directly combine with carbon and form
carbides.
Do not directly combine with carbon.
(ix) Nitrates
Decompose on heating evolving a mixture of
NO₂ and oxygen.
Decompose on heating evolving only oxygen.
(x) Solubility of salts
Sulphates, phosphates, fluorides, chromates,
oxalates, etc., are insoluble in water.
Sulphates, phosphates, fluorides, chromates,
oxalates, etc., are soluble in water.
(xi) Physical properties
Are less reactive and comparatively harder
metals. High melting points. Diamagnetic.
Soft, low melting points. Paramagnetic.
(xii) Hydration of
compounds
The compounds are extensively hydrated.
MgCl₂-6H₂O, CaCl2-6H₂0 and BaCl₂ 2H₂O
are hydrated chlorides.
The compounds are less hydrated. NaCl, KCl
and RbCl form non-hydrated chlorides
(xiii) Reducing power
Weaker, as ionisation potential values are high
and oxidation potential values are low.
Stronger, as ionisation potential values are low
and oxidation potential values are high.
