
Cosmic Chemistry:
The Modern Periodic Table
Understanding Elements
STUDENT TEXT
Chemists at Los Alamos National Laboratory, a Genesis partner organization, supply the nation
with purified chemicals. Their Web site displays a modern periodic table of the elements.
At the Web site http://mwanal.lanl.gov//CST/imagemap/periodic/periodic.html you may click on
any element and access information about its physical and chemical properties, as well as other
fascinating facts.
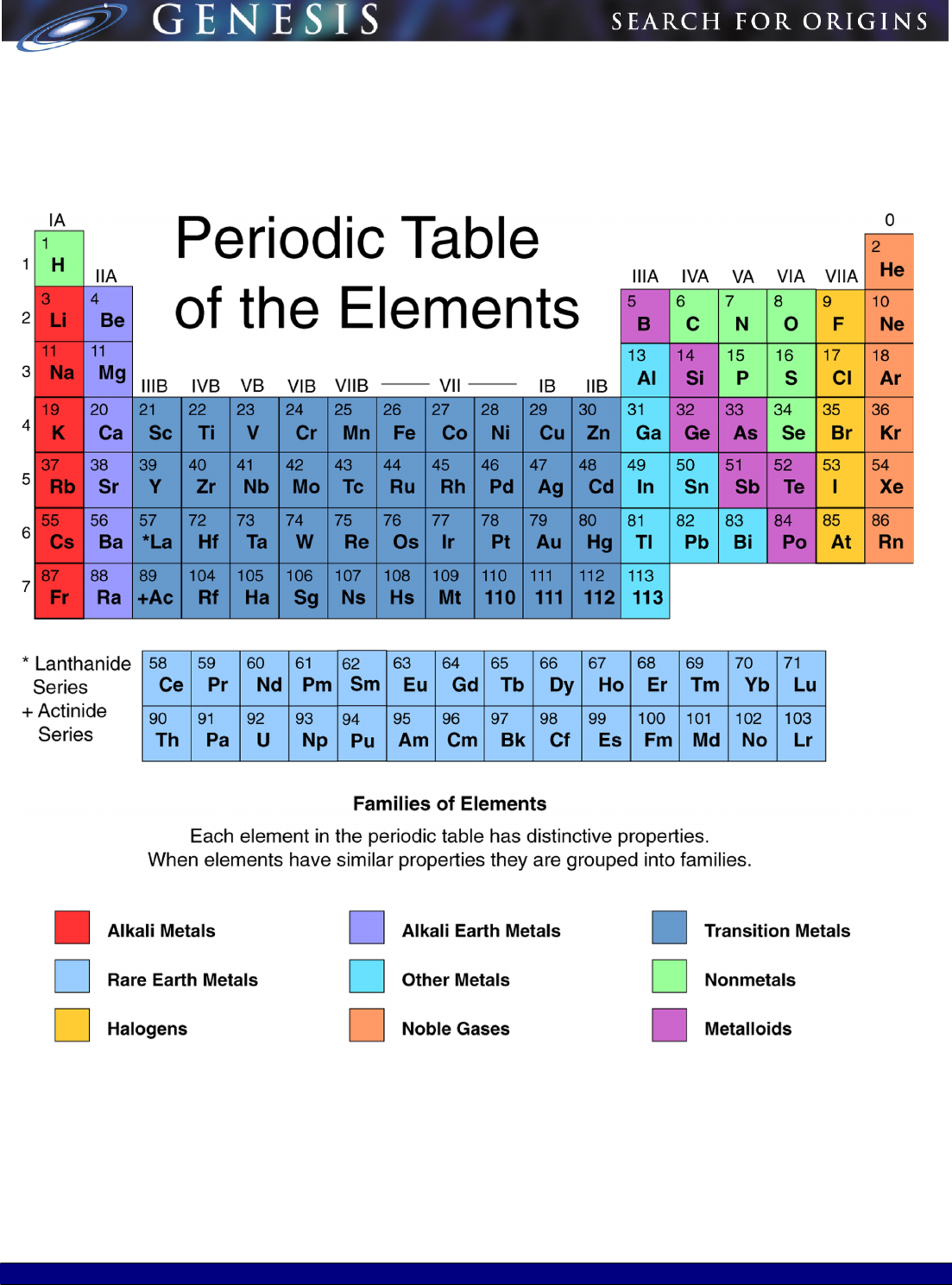
READING THE MODERN PERIODIC TABLE
Our modern day periodic table is expanded beyond Mendeleev's initial 63 elements. Most of the current periodic tables
include 108 or 109 elements.
It is also important to notice how the modern periodic table is arranged. Although we have retained the format of rows and
columns, which reflects a natural order, the rows of today's tables show elements in the order of Mendeleev's columns. In
other words the elements of what we now call a "period" were listed vertically by Mendeleev. Chemical "groups" are now
shown vertically in contrast to their horizontal format in Mendeleev's table.
Note also that Mendeleev's 1871 arrangement was related to the atomic ratios in which elements formed oxides, binary
compounds with oxygen; whereas today's periodic tables are arranged by increasing atomic numbers, that is, the number of
protons a particular element contains. Although we can imply the formulas for oxides from today's periodic table, it is not
explicitly stated as it was in Mendeleev's 1871 table. The oxides ratio column was not shown in earlier Mendeleev versions.
Can you think of a reason why not?
GROUPS
The modern periodic table of the elements contains 18 groups, or vertical columns. Elements in a group have similar
chemical and physical properties because they have the same number of outer electrons. Elements in a group are like
members of a family--each is different, but all are related by common characteristics.
Notice that each group is titled with Roman numerals and the letters A and B. Scientists in the United States and Europe
now use different titles to refer to the same groups. To avoid confusion, the Roman numerals and letters designating groups
will eventually be replaced by the numerals from one to eighteen.
PERIODS
Each of the table's horizontal rows is called a period. Along a period, a gradual change in chemical properties occurs from
one element to another. For example, metallic properties decrease and nonmetallic properties increase as you go from left
to right across a period. Changes in the properties occur because the number of protons and electrons increases from left to
right across a period or row. The increase in number of electrons is important because the outer electrons determine the
element's chemical properties.
The periodic table consists of seven periods. The periods vary in length. The first period is very short and contains only two
elements, hydrogen and helium. The next two periods contain eight elements each. Periods four and five each have 18
elements. The sixth period has 32 elements. The last period is not complete yet because new exotic or man-made
elements are still being made in laboratories.
STUDENT 1 TE XT G E NE SI S
CLASSIFICATION OF GENERAL PROPERTIES
The general properties of elements allow them to be divided into three classifications—metals, nonmetals and metalloids.
The distribution of metals is shown in your Periodic Table as boxes colored yellow, purple and two shades of blue. Metalloid
elements are in the diagonal boxes colored pink and nonmetal elements are above the diagonal line to the right of the
metalloids, in boxes colored green, gold, and red. Notice that hydrogen's box is colored green, even though it is at the top of
a group of metals.
METALS
As you can see, the vast majority of the known elements are metals. Many metals are easily
recognized by non-chemists. Common examples are copper, lead, silver and gold. In general,
metals have a luster, are quite dense, are good conductors of heat and electricity. They tend to be
soft, malleable and ductile (meaning that they are easily shaped and can be drawn into fine
wires without breaking).
All of these properties are directly related to the fact that solid metals are crystals formed from positive ions surrounded by
mobile electrons. This mobility allows electrons to absorb and reflect light in many wavelengths, giving the metals their
typical luster. It also permits electrons to absorb thermal and electrical energy from the environment or neighboring
electrons and transfer this energy to other electrons; in this way, heat and electricity can be conducted throughout the metal.
These mobile electrons hold the positive metallic ions so tightly that even when the metal sample is only a few layers thick,
as in gold foil, the sample stays intact. So, the density, malleability, and ductility of metals are also due to electron mobility.
The difference in the coloring on the Periodic Table indicates that the most metallic elements are those on the left side of
the table. The Group I Alkali Metals and the Group II Alkaline Earths have more metallic characteristics than elements
farther right whose square are colored blue, especially those that border on the metalloid elements. Generally speaking, the
most metallic metals are in the bottom left corner. As you move toward the upper right on the periodic table, elements
become less metallic in property.
ALKALI METALS
The alkali (IA) metals show a closer relationship in their properties than do any other family of elements in the
periodic table. Alkali metals are so chemically reactive that they are never found in the element form in nature.
All these metals react spontaneously with gases in the air, so they must be kept immersed in oil in the
storeroom. They are so soft that they can be cut with an ordinary table knife, revealing a very "buttery," silvery
metal surface that immediately turns dull as it reacts with water vapor and oxygen in the air. The chemical
reactivity of alkali metals increases as the atomic number increases.
Their reactions with halogens, elements in Group VIIA, are especially spectacular because some of them emit both light and
heat energy. They react with other nonmetals, albeit more slowly, forming compounds that are very stable. They also react
with acids, forming hydrogen gas and salts; with water they form hydrogen gas and metallic hydroxides, which are
sometimes called bases. They react with hydrogen to form metallic hydrides, which form strong bases in water. In all these
reactions, the metals form ionic compounds, in which each metal atom loses one electron to form a positively-charged ion or
cation.
All compounds of alkali metals are soluble in water. These compounds are widely distributed. Large mineral deposits of
relatively pure compounds of sodium and potassium are found in many parts of the world. Sodium and potassium chlorides
are among the most abundant compounds in sea water. Potassium compounds are found in all plants and sodium and
potassium compounds are essential to animal life—including human life. Lithium (Li) is the alkali metal of most interest to
Genesis scientists.
2 STUD ENT TE XT G E NE S I S

ALKALINE EARTH METALS
The alkaline earth (IIA) metals also exhibit the typical metal characteristics of high density, metallic luster
and electrical and thermal conductivity. Rocks and minerals containing silica, magnesium, and calcium
compounds are widely distributed. These chemicals are also abundant as compounds in sea water. Their
chlorides are abundant in sea water. Radium, the largest of the alkaline earths, is a radioactive element
that occurs naturally only in very small quantities. Chlorophyll, the green coloring in plants, is a
magnesium-containing compound. Calcium is a major component of animal bones, teeth and nerve cells.
Alkaline earth elements form compounds by losing, or in the case of beryllium, sharing two electrons per atom. These atoms
hold their electrons more tightly than alkali metals. They are, therefore, smaller than and not so chemically reactive as the
neighboring alkali metals. They do not require special storage because the surface of these metals reacts with air, forming a
tightly adhering layer that protects the metal and prevents additional reactions. None of them is found naturally as a free
element.
The chemical reactivity of these elements increases with size. Calcium, strontium, and barium react with water forming
hydrogen and alkaline compounds. Magnesium reacts with steam to produce magnesium oxide. Common oxides of alkaline
earth metals include lime (CaO) and magnesia (MgO), which react with water to produce strongly alkaline solutions. The
alkali metals also react readily with many other types of chemicals, including acids, sulfur, phosphorus, the halogens (Group
VIIA), and, with the exception of beryllium, hydrogen.
Alkaline earth halides are quite soluble in water. The water solubility of their hydroxides increases, but the solubility of their
carbonates and sulfates decrease with increasing atomic number. The presence of calcium and magnesium ions in water
make it "hard" because they form insoluble salts with soap. Solid calcium carbonate deposits form on container surfaces
when water evaporates. Magnesium (Li), calcium (Ca), barium (Ba), and beryllium (Be) are all of interest to Genesis
researchers.
TRANSITION METALS
The transition (or heavy) metals have most of the usual properties of metals. Their densities, which
are greater than the Group IA and IIA metals, increase and then decrease across a period. The
transition metals are also called heavy metals because their atoms are relatively small and their
large numbers of protons and neutrons give them relatively large masses.
There is a great variance in the chemical reactivity of transition metals. All the transition elements
react with halogens and most react with sulfur and oxygen. The elements from scandium through
copper form compounds that are soluble in water. The heavier elements of Group VIIB are sometimes called the platinum
metals, which, in addition to gold, are very non-reactive.
One of the main uses for transition metals is the formation of alloys—mixtures of metals—to produce tools and construction
materials for specific uses. For example, structural steel alloys, which are used in automobiles and building construction,
can contain as much as 95% iron. There are also carbon and at least six different high-alloy steels, some which contain
manganese, chromium, nickel, tungsten, molybdenum and cobalt: all transition metals.
Copper, silver and gold are sometimes known as coinage metals because they can be found naturally in the free state and
because they tarnish slowly. Since prehistoric times, they have been used in coins, utensils, weapons, and jewelry.
Although many transition metals have very high melting and boiling points, mercury (Hg) has such a low melting point that it
is a liquid at room temperature.
All the transition metals are electrical conductors, with copper, silver and gold being among the best; they vary from very
good to only fairly good thermal conductors.
3 STUD ENT TE XT G E NE S I S
Some of the transition metals exhibit colored luster and some of them are more brittle than the Group IA and IIA metals.
Whereas the compounds of the Group IA and IIA metals are white, many of the transition metal compounds are brightly
colored. Many heavy metal compounds, such as those of mercury, cadmium, zinc, chromium and copper, are poisonous.
When transition metal ions are present in even small percentages in crystalline silicates or alumina, the minerals become
gems. Rubies are gems in which small numbers of chromium ions are substituted for aluminum ions in aluminum oxide.
Chromium substitution for a small number of aluminum ions in another clear crystal, beryllium aluminum silicate, forms the
green gem known as emerald. Alexandrine may appear red or green due to chromium ion substitution in its crystal. Iron ions
can produce red garnets, purple amethysts, and blue aquamarines. Iron and titanium ions cause yellow-green peridot, and
blue turquoise gems are colored by copper ions. Titanium and chromium are two transition metals about which Genesis
scientists expect to learn a great deal.
RARE EARTH METALS
The rare earth metals consist of the lanthanide series and the actinide series. Because they are
difficult to find, they are termed rare earths. They often appear to be an add-on to the rest of the
periodic table, but actually, they should be shown in the center of the table. The table should be split
after 137 barium, and the Lanthanide series inserted. The Actinide series should be inserted after 88
radium.
(Lanthanides)
The fourteen lanthanide elements follow lanthanum (La) in the periodic table. They generally occur together in a phosphate
mineral such as monazite. They are so similar in chemical and physical properties that they are especially difficult to
separate from each other. Promethium (Pm) is unstable, and is not found in nature.
An unstable isotope of an element decays or disintegrates spontaneously, emitting various types of radiation. Another
name for an unstable isotope is a radioisotope. In some instances, the decay process is slow, with the unstable atom
lasting days or months. In others, the process is rapid, lasting tiny fractions of a second. In addition to radiation, the unstable
element changes its nucleus to become one or more other lighter elements. Approximately 5,000 natural and artificial or
manmade radioisotopes have been identified.
(Actinides)
The fourteen actinides follow actinium (Ac) in the periodic table. They are all unstable, and most do not occur in nature.
Less is known about these elements than about any other family, since some of them have only been produced in tiny
quantities. Uranium (U) is the most well-known naturally occurring member of this group of elements. Mendelevium (Md),
element number 101, is named for Dmitri Mendeleev, the Russian chemist who first arranged the elements in a table in
order of increasing atomic mass. Examining radioactive nuclei in solar wind is one of the measurement objectives of the
Genesis mission.
OTHER METALS
Other metals include heavier elements of Groups IIIA, IVA, and VA. They form a staircase inside the
periodic table. The metals in Group IIIA are aluminum (Al), gallium (Ga), indium (In), and thallium (Tl).
The metals in Group IVA are tin (Sn) and lead (Pb), and the only metal in Group VA is bismuth (Bi).
As atomic number decreases within each group, their metallic character gets weaker. For instance,
boron (B), above aluminum in Group IIIA, is a metalloid rather than a metal. Aluminum, tin, and lead
are readily recognized as metals by non-chemists.
Aluminum (Al) is the only true metal in Group IIIA. Aluminum ores are found in great abundance in the Earth’s crust, but the
metal’s manufacture by an electrochemical process from bauxite ore requires ten times the energy needed to produce steel.
Because aluminum is light, strong, very malleable and resists corrosion, it is an especially good industrial and construction
metal.
The principal use of tin (Sn) is as a corrosion-resistant coating for iron, although it is widely used in alloys, such as bronze
and solder. Lead (Pb) is the principal constituent of lead storage batteries, but it is also used to form solder. Its use in paints
4 STUD ENT TE XT G E NE S I S
has been discontinued because of its toxic effect on the human body. Bismuth (Bi) is hard and brittle; it is used in alloys with
low melting points that are used for electrical safety appliances.
METALLOIDS
The metalloids include boron (B), silicon (Si) and germanium (Ge), arsenic (As) and antimony
(Sb), tellurium (Te) and polonium, (Po). Note that they are arranged in stair steps between the
metals and nonmetals.
Metalloids have some of the properties of metals and nonmetals—and each metalloid has its own
unique mixture. A few are shiny like metals, but do not really have a metallic luster. Some
metalloids have very high melting and boiling points; others do not. Others conduct electricity—but
their electrons are mobile in only certain directions, so they are called semi-conductors. This
makes them useful in designing transistors and other solid state electronic components. Genesis
scientists are interested in boron because the collection wafer material is pure silicon.
NONMETALS
The nonmetallic elements are in the upper right portion of the Periodic Table. At room temperature and pressure, many of
them exist as gases, but one is a liquid. Others are either the hardest or the softest of solids. The nonmetals have few
chemical properties in common. They range from fluorine, the most active nonmetal, to the most nonreactive of the
elements, the noble gases. Millions of compounds formed from carbon, hydrogen, oxygen, sulfur and nitrogen are known as
organic chemicals.
Oxides of sulfur and nitrogen have been identified as atmospheric pollutants. Nonmetallic compounds also include salts as
well as many acids and bases. Many of these salts are found in soil or dissolved in ocean water. Any ions formed by
nonmetals are negatively charged.
Almost eighty percent of our atmosphere is made up of nitrogen gas and most of the rest is oxygen, which is necessary for
human respiration and metabolism. There are negligible amounts of noble gases in our atmosphere.
Many of the nonmetals are colored, including yellow sulfur, red and yellow phosphorus, yellow-green fluorine, pale yellow
chlorine, red-brown bromine, and violet-black iodine. Others, like oxygen, nitrogen, and the noble gases are colorless. Only
sulfur is found as a free element in nature.
Some of the nonmetals are molecular, such as the diatomic halogens, nitrogen, and
oxygen; phosphorus forms molecules of four atoms and sulfur is found in rings of eight
atoms. The noble gases exist as monoatomic gases. On the other hand, any sample of
carbon, whether it be the graphite in your pencil lead or a diamond, is one large
molecule of carbon atoms.
If metal atoms are closely packed like stacked building materials, leading to high
densities, then the low density of nonmetals is like the same building materials widely
distributed with open spaces between them in the constructed building. Electrons in the crystalline structures of nonmetallic
solids are tightly held in chemical bonds; so, nonmetals are notably good electrical and thermal insulators.
HALOGENS
The halogens are the gases in Group VIIA of the periodic table. Their name indicates that they are salt-formers. This is
appropriate since they react easily with other elements, especially the alkali and alkaline earth elements in the left columns
of the periodic table. They are all considered highly reactive elements.
Chlorine (Cl) is one of the halogens. It is a highly poisonous yellow and green-colored gas. It combines easily with an
explosive alkali metal called sodium (Na) to form a compound called sodium chloride. The compound’s chemical formula is
NaCl, and it goes by the common name of table salt.
5 STUDENT TEXT GENESIS

Actually the term salt is used in a more general sense to mean any compound of a halogen with an alkali metal or alkaline
earth metal. These can combine in many different arrangements. Some types of salts include magnesium bromide (MgBr
2
)
and potassium iodide (KI).
Except for the noble gases, the chemical reactivity of nonmetals decreases as the size of the family member increases.
Fluorine is the most electronegative of the elements, which means that it has the most attraction for a pair of electrons
being shared. It is so reactive that metal flakes, glass, and even water will react brightly in it, so it requires special handling
and great care in its use and storage.
Melting points and boiling points increase as the size of the atom in the family increases. Note the physical states of the
halogens. Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid at room temperatures. Astatine is a
radioactive member of the halogen family and radon has some radioactive isotopes that are dangerous to human health.
Fluorine (F), the lightest halogen, is also the most reactive element on Earth. It is also the most electronegative, having the
ability to steal electrons from other elements easily. This dangerously reactive element requires special handling and great
care in its use and storage.
NOBLE GASES
The noble gases were unknown in Mendeleev's time. The lightest and most stable of
these gases, helium, was discovered from its bright yellow solar spectral line in 1868.
The existence of helium on Earth was not discovered until 1895. The noble gases are
Group 0 in the periodic table.
These elements are termed noble because they do not interact with other elements to
form compounds. Another way to say this is that they are inert. Their atoms do not even interact with each other, so they
exist as mono atomic gases.
Examining noble gas elements and isotopic ratios in solar wind is a major science objective of the Genesis mission. The foil
collectors returned from the moon on Apollo missions provided precise solar wind helium (He) and neon (Ne) isotope ratios
not previously known. One surprise was a
20
Ne/
22
Ne ratio that was 38% higher than that of samples of Earth’s atmosphere.
The results of the Genesis mission may help scientists better understand the solar system’s diversity of noble gas
distribution.
HYDROGEN
Chemists are not in agreement about the placement of hydrogen on the periodic table. In the periodic table in this module,
hydrogen is shown as a nonmetal, but placed above Group 1A metals, because it also exhibits some chemical properties
similar to those metals. It exists in the free state as diatomic molecules and it reacts with active metals in much the same
manner as the halogens. But it also found bonded to other nonmetals in organic compounds.
Hydrogen is the most abundant element in the universe. It is estimated that ninety percent of the atoms in the universe are
hydrogen atoms. The sun and other stars appear to be composed largely of hydrogen, as do the gases found in interstellar
space.
Hydrogen is a component of more compounds than any other element and makes up 11% of the mass of water, its most
abundant compound. It is found in only negligible quantities uncombined on the Earth, even though it is the ninth most
abundant element in the Earth's crust.
Hydrogen is the principal energy source in the Sun's high temperature nuclear fusion reaction. This major nuclear reaction,
which occurs at temperatures in excess of 40,000,000°C, is thought to involve the combination of the nuclei of a deuterium
atom and a tritium atom to form a helium nucleus and a neutron.
6 STUDENT TEXT GENESIS
In examining the modern periodic table, one notices the chemical elements arranged in groups and periods. They are
classified by their general physical and chemical properties into their groups: metals, metalloids, and nonmetals, which can
be further subdivided. These classifications help chemical researchers understand known elements and predict the
properties of new manmade elements.
7 STUDENT TEXT GENESIS
