
Periodic Activity of Metals
Periodic Trends and the Properties of the Elements
Introduction
Elements are classified based on similarities, differences, and trends in their properties, including their chemical reactions.
The reactions of alkali and alkaline earth metals with water are pretty spectacular chemical reactions. Mixtures bubble and
boil, fizz and hiss, and may even smoke and burn. Introduce the study of the periodic table and periodic trends with this
exciting demonstration of the activity of metals.
Concepts
• Alkali and alkaline earth metals • Periodic table and trends
• Physical and chemical properties • Metal activity
Materials
Calcium turnings, Ca, 0.3 g Beaker, Berzelius (tall-form), Pyrex
®
, 500-mL, 4
Lithium metal, Li, precut piece Forceps or tongs
Magnesium ribbon, Mg, 3-cm Knife (optional)
Sodium metal, Na, precut piece Petri dishes, disposable, 4
Phenolphthalein, 1% solution, 2 mL Scissors
Water, distilled or deionized, 600 mL
Safety Precautions
Lithium and sodium are flammable, water-reactive, corrosive solids; dangerous when exposed to heat or flame. They react violently
with water to produce flammable hydrogen gas and solutions of corrosive metal hydroxides. Hydrogen gas may be released in sufficient
quantities to cause ignition. Do NOT “scale up” this demonstration using larger pieces of sodium or lithium! These metals are shipped
in dry mineral oil. Store them in mineral oil until immediately before use. Do not allow these metals to stand exposed to air from one
class period to another or for extended periods of time. Purchasing small, pre-cut pieces of lithium and sodium greatly reduces their
potential hazard. Calcium metal is flammable in finely divided form and reacts upon contact with water to give flammable hydrogen
gas and corrosive calcium hydroxide. Magnesium metal is a flammable solid and burns with an intense flame. Perform this
demonstration in a well-ventilated lab only. Do not handle any of the metals with bare hands. Wear chemical splash goggles, chemical-
resistant gloves, and a chemical-resistant apron. All students or spectators should also be wearing chemical splash goggles
during this demonstration. Use a Class D powder fire extinguisher such as dry sand for reactive metals. Please review current
Material Safety Data Sheets for safety, handling, and disposal information.
Preparation
1. Obtain four 500-mL, tall-form beakers and label them Li, Na, Mg, and Ca. Add approximately 150 mL of distilled or
deionized water to each beaker. Label four Petri dishes Li, Na, Mg, and Ca and place them next to the beakers.
2. Cut the magnesium ribbon into 3-cm strips using scissors.
3. The precut lithium and sodium metal pieces should be approximately 0.5 cm × 0.5 cm × 0.5 cm, or 0.2–0.3 g each.
4. Divide the calcium turnings into five samples, about 0.3 g each.
Procedure
1. Place one piece of each metal in its respective Petri dish on an overhead projector. Observe and compare the physical
properties of the metals: Color, luster (shine), hardness, and malleability.
2. Have students record the properties of the elements on the worksheet.
© 2016 Flinn Scientific, Inc. All Rights Reserved. 1
Publication No. 95022
061616
SCIENTIFIC
Periodic Activity of Metals continued
2
© 2016 Flinn Scientific, Inc. All Rights Reserved.
3. Discuss possible “rankings” of the metals with respect to their physical properties. Which metal appears to be the shiniest?
Softest (or hardest)?
4. Use forceps or tongs to quickly transfer one piece of sodium metal to water in its respective labeled (Na) beaker. Have
students make detailed observations of the resulting chemical reaction and record all observations on the worksheet. Sodium
metal, which initially floats on the surface, immediately begins to “hiss and sizzle” on the water surface and appears to melt. Popping
sounds are heard and the metal begins to bounce around and finally disappear. A smoky gas forms and ignites the metal on the surface of
the water. Sparks may be seen where the metal ignites.
5. Repeat step 4 using lithium metal in its respective (Li) beaker. As observations are made, ask students to compare the rate
and intensity of the reaction versus that of sodium. Record all observations on the worksheet.
6. Repeat step 4 twice more, using calcium and magnesium, respectively. Compare the activity of each metal against the
previous metal and against sodium as a reference metal. Record all observations
7. Refer to the observations to rank the metals in order of their reactivity. Which metal is most active? Least active? Answer
Questions #1 and #2 on the worksheet.
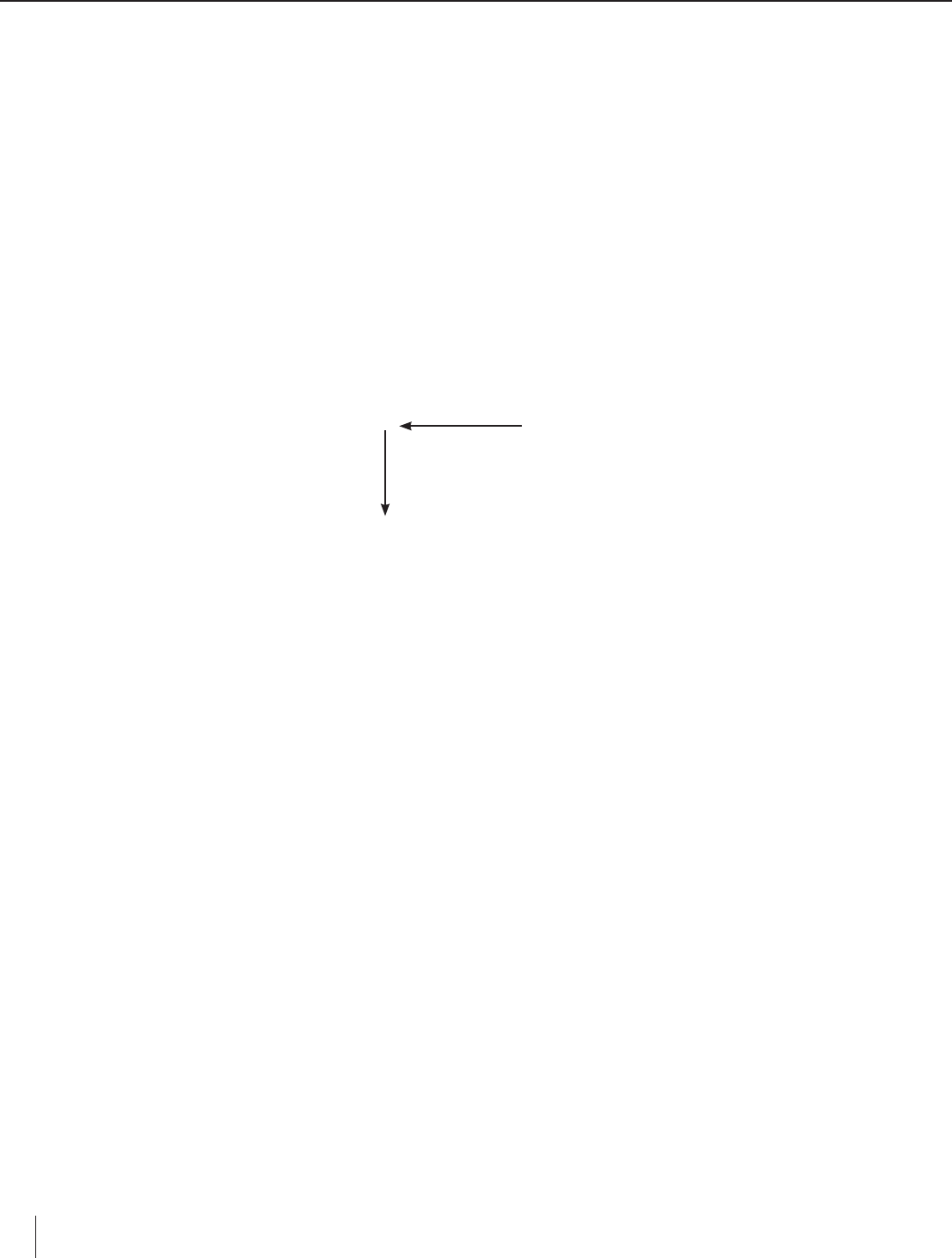
8. Refer to Question #3 on the worksheet. Based on class discussion, draw arrows to indicate the directions in which metal
activity increases across a row and within a column in the periodic table, as shown below.
Activity increases
Li
Na Mg
Ca
9. Ask students to predict the activity of potassium metal based on the observed periodic trend in the activity of metals
(Answer Question #4 on the worksheet). Discuss the extreme reactivity of potassium metal (and why it was not used in this
demonstration).
10. After the metals have reacted (with the exception of magnesium, which does not react under these conditions), add 5 drops
of phenolphthalein solution to the mixture in each beaker.
11. Observe the color change(s) and discuss what a color change indicates. What types of solutions exhibit this color change?
Discuss the possible identity of the product(s). (See the Discussion section.)
12. Write balanced chemical equations for the reactions of the metals with water (Question #5). Discuss the evidence for the
formation of both hydrogen gas and metal hydroxides.
13. (Optional) Have students write a paragraph describing in words the physical and chemical properties of one of the metals.
Instruct students to include as much descriptive detail as possible. An example is given below.
“Sodium is a soft, silver-white solid. Upon exposure to air it gradually develops a white oxide coating. It can be cut with a
knife. It is less dense than water and reacts spontaneously and vigorously on contact with water. The metal piece appears to
pop or sizzle on the surface and a smoky white gas forms. The metal may ignite on the surface of the water in the vicinity
of the smoke. The products are hydrogen gas and sodium hydroxide. The hydrogen gas that is formed ‘pops’ and briefly
ignites. Sodium hydroxide makes the solution basic (red) to phenolphthalein indicator.”
Disposal
Please consult your current Flinn Scientific Catalog/Reference Manual for general guidelines and specific procedures governing the
disposal of laboratory waste. Use tongs or forceps to remove unreacted magnesium from its beaker. Dispose of excess magnesium
metal according to Flinn Suggested Disposal Method #26a. Do not dispose of any of the other reaction mixtures until all
of the metal in each has completely reacted. The resulting basic solutions in each beaker can be neutralized and disposed of
according to Flinn Suggested Disposal Method #10.

Periodic Activity of Metals continued
3
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Tips
• We strongly recommend the purchase of small quantities only of sodium and lithium metal. Purchase only the
amounts that will be used in one academic year. Sodium and lithium are available in small, precut pieces that are
suitable for demonstrations (Flinn Catalog Nos. S0329 and L0057, respectively).
• We do not recommend the use of potassium in the high school science laboratory. Potassium is considerably more water-
reactive than sodium and is a serious fire and explosion hazard. There is a significant and often undetectable explosion
risk because of the propensity of potassium to form a superoxide (peroxide) coating on its surface. Potassium reacts with
oxygen in air to form a coating of yellow potassium superoxide (KO
2
), even when the metal is stored under dry mineral
oil. Old pieces of potassium are thus extremely dangerous. When the metal is cut, the pressure of the knife may touch off
a violent, uncontrollable, explosive reaction between the superoxide coating and the underlying metal.
• Calcium metal must be reasonably fresh to react with water. Old (dull) calcium metal will not react with water.
• The reactions of sodium and lithium with water may be quite vigorous—we recommend using tall-form (Berzelius)
beakers to contain any molten metal pieces that may splatter. Do NOT scale up this demonstration.
• The use of a ChemCam
™
video camera will make it easier for students to observe the appearance and properties of the
metal pieces.
• Demonstrate the softness of lithium and sodium by showing how the metal pieces can be cut with a dry spatula or knife.
• In ranking the metals in order of their activity, it is easier to begin with pairwise comparisons. Which is more active—
sodium or lithium? Calcium or magnesium? Magnesium or sodium?
• Is the activity of metals related to their hardness? Density? The answer, a firm “maybe,” depends on the comparisons
being made. The alkali metals as a group are softer and less dense than their nearest alkaline earth metal neighbors, and
also more reactive. Within the group of alkali metals, however, the opposite trend is observed. Lithium is less dense but
also less reactive than sodium.
Discussion
Sodium reacts with water to form hydrogen gas and sodium hydroxide, according to the following balanced chemical equation.
2Na(s) + 2H
2
O(l) → H
2
(g) + 2NaOH(aq) + Heat
As sodium metal reacts with water, a great deal of heat is generated. The sodium melts and seems to float or bob on the water
surface. The oxide coating that may have dulled the metal disappears and sodium’s silvery gray, metallic character is more
apparent. The evolution of hydrogen gas is evident in the production of a white smoke, which pops and ignites as it is heated
above its flash point. The formation of sodium hydroxide, a strong base, is inferred from the color change observed with
phenolphthalein, an acid–base indicator. Phenolphthalein is colorless in neutral or slightly basic solutions (pH < 8) and red in
more basic solutions (pH >10). Between pH 8 and 10 phenolphthalein appears various shades of pink.
The balanced chemical equations for reactions of other active metals with water are given below.
2Li(s) + 2H
2
O(l) → H
2
(g) + 2LiOH(aq)
Ca(s) + 2H
2
O(l) → H
2
(g) + Ca(OH)
2
(aq)
Of the four metals tested, sodium is the most active and magnesium is the least active. Magnesium does not react with water
under these conditions (it may react slightly in hot water). The order of metal reactivity is Na > Li, Ca >> Mg. Periodic trends
in the activity of metals are generally attributed to differences in their ionization energies. The activity of metals increases as
the value of their first ionization energy decreases. Within a vertical column (group or family) of elements in the periodic table,
ionization energy decreases from top to bottom. As a result, metal activity increases going down a column in the periodic table
(K > Na > Li; Ca > Mg). Across a horizontal row (period or series) in the periodic table, ionization energy also increases from
left to right. As a result, metal activity decreases from left to right across a row in the periodic table (Na >> Mg; K >> Ca).
Periodic Activity of Metals continued
4
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Connecting to the National Standards
This laboratory activity relates to the following National Science Education Standards (1996):
Unifying Concepts and Processes: Grades K–12
Evidence, models, and explanation
Constancy, change, and measurement
Content Standards: Grades 5–8
Content Standard A: Science as Inquiry
Content Standard B: Physical Science, properties and changes of properties in matter, transfer of energy
Content Standards: Grades 9–12
Content Standard A: Science as Inquiry
Content Standard B: Physical Science, structure of atoms, structure and properties of matter, chemical reactions, inter-
actions of energy and matter
Answers to Worksheet Questions
Data Table
Physical Properties of Metals — Observations
Lithium
Dark gray–black solid. The surface has
a slight bluish sheen and a “tacky” tex-
ture, like hard rubber. Can be cut with
a knife.
Magnesium
Silvery gray, shiny, hard metal “ribbon.”
The metal can be easily bent and cut
with scissors. Light weight.
Sodium
Soft, silver–white, shiny metallic solid.
Can be cut with a knife.
Calcium
Shiny, dark silver–gray metal pieces;
hard—not easily cut. Rough texture.
Reactions of Metals with Water — Observations
Lithium
Lithium floats on water—reacts slowly
at first, then more vigorously. “Sizzles”
and bounces on water. Bubbles of gas,
smoky fumes observed as metal turns
white around edges and then “disap-
pears” after about 5 minutes.
Magnesium
Magnesium does not react with water at
room temperature.
Sodium
Sodium floats on the water—immedi-
ately begins to “sizzle” and hiss. Bubbles
of gas, heat released. Sodium melts
and “bounces” around. Sparks as gas or
metal ignites. The reaction is very fast—
metal disappears within 30 seconds.
Final solution is slightly cloudy.
Calcium
Metal sinks, then mixture slowly begins
to bubble, sizzle and fume. Metal turns
white and melts and water becomes
very cloudy.
Periodic Activity of Metals continued
5
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Questions
1. What is the common name for the family of metals in (a) Group 1 and (b) Group 2 of the periodic table?
(a) Alkali metals (b) Alkaline earth metals
2. Rank the four metals used in this demonstration from most active to least active based on their reactivity with water.
Na > Li > Ca >> Mg
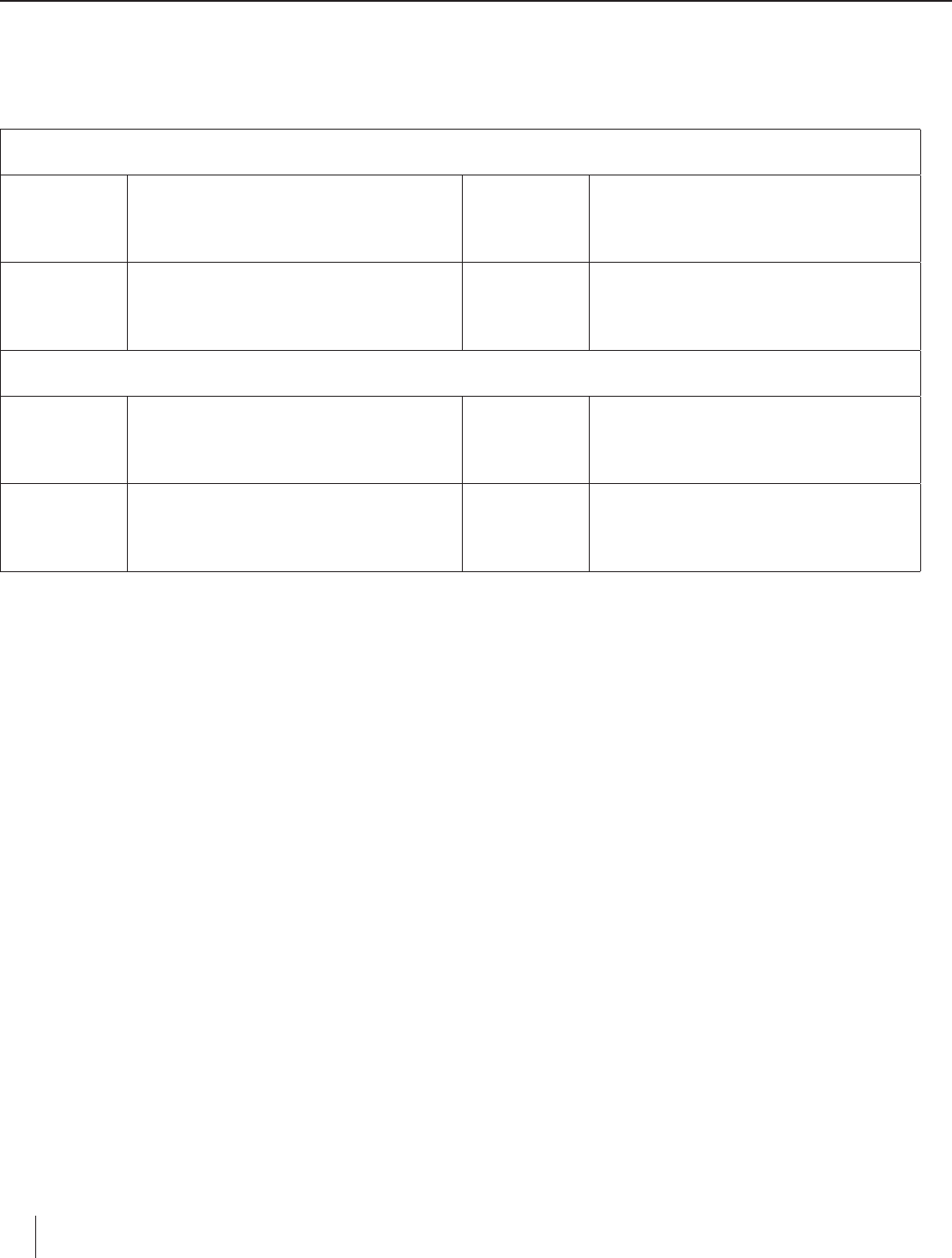
3. The metals are arranged below according to their relative positions in the periodic table. (a) Draw a horizontal arrow across
the top to show the direction in which the activity of a metal increases across a period (row) in the periodic table. (b) Draw
a vertical arrow along the side to show the direction in which the activity of a metal increases within a group (family) in the
periodic table.
Group 1 Group 2
Period 2 Li Metal activity increases in the directions the arrows point.
Period 3 Na Mg
Period 4 K Ca
4. Look up the position of potassium metal in the periodic table and write in the symbol for potassium in the appropriate
location in the arrangement of metals in Question #3. Based on the trend in metal activity observed in this activity, predict
whether potassium metal is more or less reactive than sodium with water.
Potassium metal is dangerously reactive with water (and air). It is more reactive than both sodium and calcium. Metal activity increases
going down a column in the periodic table.
5. Write a balanced chemical equation for the reaction of (a) sodium metal and (b) calcium metal with water.
a. 2Na(s) + 2H
2
O(l) → 2NaOH(aq) + H
2
(g)
b. Ca(s) + 2H
2
O(l) → Ca(OH)
2
(aq) + H
2
(g)
Reference
This activity was adapted from a demonstration in The Periodic Table, Volume 4 in the Flinn ChemTopic
™
Labs series, Cesa, I.,
Editor; Flinn Scientific: Batavia, IL (2002).
Flinn Scientific—Teaching Chemistry
™
eLearning Video Series
A video of the Periodic Activity of Metals activity, presented by Irene Cesa, is available in Periodic Trends and the Properties of the
Elements, part of the Flinn Scientific—Teaching Chemistry eLearning Video Series.
Materials for Periodic Activity of Metals are available from Flinn Scientific, Inc.
Materials required to perform this activity are available in the Periodic Activity of Metals—Chemical Demonstration Kit available
from Flinn Scientific. Materials may also be purchased separately.
Catalog No. Description
AP7180 Periodic Activity of Metals—Chemical Demonstration
Kit
GP1060 Beaker, Berzelius, 500-mL
C0345 Calcium 10 g
L0024 Lithium, 10 g
M0139 Magnesium Ribbon, 12.5 g
S0329 Sodium, 5 pieces
Consult your Flinn Scientific Catalog/Reference Manual for current prices.
Periodic Activity of Metals continued
6
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Periodic Activity of Metals Worksheet
Data Table
Physical Properties of Metals — Observations
Lithium Magnesium
Sodium Calcium
Reactions of Metals with Water — Observations
Lithium Magnesium
Sodium Calcium
Questions
1. What is the common name for the family of metals in (a) Group 1 and (b) Group 2 of the periodic table?
2. Rank the four metals used in this demonstration from most active to least active based on their reactivity with water.
3. The metals are arranged below according to their relative positions in the periodic table. (a) Draw a horizontal arrow
across the top to show the direction in which the activity of a metal increases across a period (row) in the periodic table. (b)
Draw a vertical arrow along the side to show the direction in which the activity of a metal increases within a group (family)
in the periodic table.
Group 1 Group 2
Period 2 Li
Period 3 Na Mg
Period 4 Ca
4. Look up the position of potassium metal in the periodic table and write in the symbol for potassium in the appropriate
location in the arrangement of metals in Question #3. Based on the trend in metal activity observed in this activity, predict
whether potassium metal is more or less reactive than sodium with water.
5. Write a balanced chemical equation for the reaction of (a) sodium metal and (b) calcium metal with water.
